Synthesis and Characterization of ZnO Nanoparticles for Biomedical Applications-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
The development in the field of nanoscience and nanotechnology has opened new avenues for large number of technological applications in the field of biological and biomedical science. The application of nanotechnology in medical applications is comes under "nanomedicine" category which introduces new tools, devices and therapies for treatment of disease. Till date extensive work has been going on the drug delivery, medicine target and used for the treatment of cancer. Due to large surface area to volume ratio of NPs, the loading capacity of therapeutic agents for delivery to target cells and antibacterial activity. By improving UV illumination factors, ZnO particle size, concentration, morphology, and surface modification, powerful antibacterial results would be obtained ZnO NPs exhibit antidiabetic effects also with improved glucose tolerance [1-10]. ZnO is also a biocompatible material having high Isoelectric Point (IEP) of about 9.5 which makes it suitable for absorption of proteins with low IEPs where the protein immobilization is primarily driven by electrostatic interaction.
ZnO nanostructures exhibit high specific surface area, nontoxicity, chemical stability, electrochemical activity, and high electron communication features. Such properties forecast the potential applications of ZnO and the promising material for biosensor applications. ZnO generally exhibits n-type conductivity with the electrons in its valence band as charge carriers. The structure of ZnO, consists of a number of alternating planes composed of tetrahedrally coordinated O2- and Zn2+ ions, stacked alternately along the c-axis. In ZnO, zinc is acting as a deep acceptor and oxygen is acting as a deep donor. ZnO exhibits variety of nanostrustructres e.g. nanohelices/ nanosprings, seamless nanorings, aligned nanopropellers, patterned growth of nanowires, mesoporous single-crystal nanowires, ultra narrow ZnO nanobelts and polyhedral cages etc. Recently, biosafe-ZnO materials have been used for curing and diagnosing deadly diseases.
In this work, ZnO NPs were prepared by chemical coprecipitation method using zinc acetate dihydrate precursor and analyzed their structural microscopic and optical properties by high resolution XRD, SEM, FT-Raman and UV-VIS spectroscopic techniques. Characterization of these NPs becomes important in deciding their device worthiness before integrating them in the device for the cost effectiveness, consistency and reliability of the sensor or as drug delivery agent.
Experimental Measurements
Materials used
Zinc acetate dehydrate and sodium hydroxide analytical reagent (AR) grade chemicals of e-Merck were used without any further purification for synthesis of ZnO NPs.
Synthesis of ZnONPs
1.00M NaOH was added to 0.5M zinc acetate dehydrate aqueous solutions dropwise with constant stirring for precipitating zinc oxyhydroxide. The precipitated NPs were filtered, dried at 70 oC and sintered at 450 oC to obtain to oxides of nanoparticles [11].
The as-prepared NPs were characterized by Bruker AXS D8 Advance diffractometer which has in built Diffracplus software using CuKα radiation for structural details, LE0-440 SEM with EDS attachment, Perkin Elmer GX 2000 FT Raman and Shimazu UV-VIS spectrometers were for morphological, structural and optical characterization of these films.
Results and Discussion
ZnO NPs were formed through three step chemical reaction mechanism. It proceeds via the process of hydrolysis, condensation and poly-condensation.
(i) Zinc acetate on dissolving in water is partially hydrolyzed and the rest is ionized. The extent of hydrolysis depends upon the availability of water from the ambient atmospheric humidity.
(ii) The hydrolysis of zinc acetate leads to the formation of the basic zinc acetate, which on evaporation of the water, does not give pure zinc acetate but produces a mixture of zinc acetate and basic zinc acetate.
(iii) Two molecules of Zn(0H)2 condense. After the evaporation of the water molecules, this would result in a final product which can be written as HO (Zn-O-Zn) n OH where n is the number of molecules taking part in the condensation process (poly-condensation).
XRD pattern of ZnO NPs is presented in Figure 1(a) which shows diffraction peaks at 2Ө (in degrees) 31.680, 34.340, 36.180, 56.540, 62.780 and 68.260 of (100), (002), (101), (110), (103) and (112) planes respectively. The calculated parameters of unit cell are a=0.3255(0.0004)nm and c=0.5216(0.0008) nm. These are very close to the reported PDF#36-1451 data (a0=0.3250nm and c0=0.5207nm). The crystallite size of ZnO NPs was measured by using Sherrer formula and calculated from (101) peak in the range of 20 to 30nm. SEM micrograph of ZnONPs is shown in Figure 1(b). SEM micrograph revealed the presence of variable sized and at some regions agglomerated nanoparticles. EDS spectrum showed strong peaks of Zn due ZnKa (8.64keV) and ZnKβ (9.57keV) excitations and the peak for oxygen are observed. FT Raman spectrum Figure 1(c) showed A1, E1 and E2 phonon and two phonon bands of ZnO similar to bulk ZnO (16-19) by considering C6v point group and four molecules per unit cell. In these ZnO NPs Raman spectrum, E2 (Low) and E2 (High) phonon peaks are observed at 108.9 and 434.46cm-1, E1(LO) mode at 583.32cm-1 and A1 (TO, LO) modes at (383.49, 574.84) respectively.
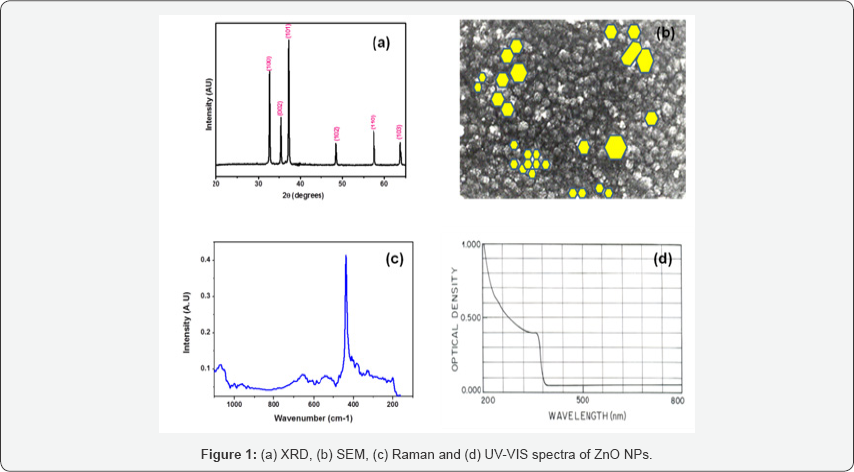
In addition to these phonon peaks, two phonon peak of 2E2 at 215.43cm-1, 3E2 at 346.72cm-1, (E2+ A1(LO)) at 676.23cm- 1, (E1 (LO)+A1(TO)) mode at 955.08cm-1 and (E1(TO)+A1(LO)) mode at 998.98 cm-1. UV-VIS spectrum of ZnO NPs recorded in 200-800nm region as presented in Figure 1(d) shows exciton absorption peak is at 373nm i.e. close to the expected value 378nm of ZnO (20,21). These characterization studies revealed the successful synthesis of pure zinc oxide nanoparticles without any impurities and unreated excessive precusor. One can easily use them in bimedical applications as sensing electrode or in drug delivery application. The functional groups present on medicine can easily bind with ZnO NPs through weak hydrogen bonding or form chemical bond through chemical reaction which on administration in body will be easily released to cure infected region of organ or kill demaged cells. During this process, pH of infected cells/organs vary from high to medium to low in aqueous form [12]. In future, the setup will be developed for the application of these prepared ZnO NPs in biosensor application.
Conclusion
ZnO NPs were grown by chemical co-precipitation route. XRD, SEM, EDS, FT Raman and UV-VIS optical studies revealed the uniform, polycrystalline, wurtzite structure with preferential orientation of (101) plane of these nanoparticles. Raman phonon spectra exhibited the change in crystalline field and existence of residual stress in the film network. UV-VIS spectra showed excitons excitation at 373nm.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555580.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment