Zno Nanoparticles in Hydrogel Polymer Network for Bio-Imaging-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
Luminescent II-VI semiconductors have been studied immensely for their optoelectronic and bio-photonic applications. Semiconductor emitters are becoming more prominent in bio medical imaging because of their narrow, tunable emission range, and high stability. Thus by using semiconductor markers the emission wavelength may be tuned to minimize noise from auto fluorescence of biological samples, and semiconductors do not photo-bleach [1]. Zinc oxide (ZnO) is a direct band gap II-VI semiconductor with wide band gap energy of 3.37 eV at room temperature and a high exciton binding energy of 60 meV [2]. Since the exciton binding energy in ZnO is greater than the thermal energy (27 meV), emission at biocompatible temperatures are unlikely from thermally induced auto-fluorescence in biological samples. Excitons in ZnO are very stable at room temperature which ensures that its luminescence will not be altered by thermal energy and has stable UV emission [2]. ZnO also has low toxicity [3], is highly photostable [4], and is a biodegradable material [5], which makes it a potentially effective bio-material for bioimaging applications and biomedical engineering. Under normal conditions, the ZnO nanoparticles dispersed in solution precipitates under gravity and therefore it is a challenge to disperse ZnO nanoparticles homogenously in water for biological imaging. By encapsulating nanoparticles in hydrogel based polymer matrix, a micro-gravity environment can be created due to the density matching of hydrogel with water for the dispersion of ZnO in solution. In this work, a novel material hybrid ZnO system is synthesized for biological imaging.
Poly N-Isopropylacrylamide (PNIPAM) hydrogels are novel nanostructures which react to environmental stimuli such as temperature [6], pH [7], electric field [8], magnetic field [9], and organic solvents [10]. They are ofparticular interest in bio-medical applications since they are water based and bio-compatible. PNIPAM hydrogels have found applications in drug delivery [11], cell adhesion mediators [12], and precipitation of proteins [13]. Poly N-isopropylacryl-amide (PNIPAM) is a polymer which exists in two phases (hydrophobic and hydrophilic) and its volume and refractive index changes as the solution temperature crosses the lower critical solution temperature (LCST). This phase change is reversible and does not affect the stability of the polymer. In this paper, the encapsulation of ZnO nanoparticles within a hydrogel network is optimized for enhanced photo-stability needed for biological imaging. The optical property of the photostable ZnO nanoparticles used for bioimaging has been characterized using time-resolved photoluminescence spectroscopy. The use of the ZnO nanoparticles for imaging of plant cell has also been demonstrated.
Material Synthesis
ZnO nanoparticles were synthesized under various conditions using gas evaporation method or arc plasma technique with an arc current of 90 amperes. No catalyst or precursors were used in this method. The samples were synthesized at atmospheric pressure and are oxygen rich in nature. Raman spectroscopy and X-ray diffraction analysis revealed the presence of a significant amount of hydrogen along with the formation Zn(OH)2 complex. The presence of a C-O complex in the ZnO the nanocrystals was also confirmed from the 1057cm-1 Raman peak. The presence of the Zn (OH)2 and C-O complex influences the cross-linking of the ZnO nanocrystals with the polymer network. ZnO is insoluble in water and because biological applications require water soluble materials, ball milling method is employed to disperse ZnO nanoparticles in water. Ball milling is a non traditional method but is an effecting technique for the homogenization of the nanoparticles in solution. It uses a type of grinder called ball mill for grinding or mixing materials like ores, chemicals and metallic powders. Typically aluminum balls are used as the grinding media in this study. Suspension of ZnO nanoparticles in de-ionized water with Al balls is mixed in an air tight cylindrical shaped ceramic jar (ball mill). This jar is placed on top of two drive shafts and rotated (rolled) for 50 hours at a speed of 600rpm. Smaller nanoparticles have a higher probability to disperse in water, while larger nanoparticles tend to precipitate out. Pure dispersion of ZnO in water is separated from the precipitate. The major limitation of this method is that one cannot determine the concentration of ZnO in the dispersion without electron microscopy. Dispersing the nanoparticles in a solvent also reduces the agglomeration of powder ZnO, and can be easily incorporated in hydrogel matrix than ZnO powder. The particle formed after ball milling is shown in Figure 1a. The stability of ZnO nanoparticles were dispersed in water using ball mill method is unpredictable. Therefore, Poly n-isopropyl acrylamide (PNIPAM) hydrogel colloidal solution is used to modify the surface of ZnO for better stability and bio-compatibility.

PNIPAM hydrogel is synthesized with N-isopropylacrylamide (NIPAM) as the main monomer using the precipitation polymerization method. The schematic is shown in Fig.1b. The monomer along with the cross linkers and co-monomer were mixed in 230ml of water. The oxygen was removed from the mixture by purging with nitrogen and was heated to 60degrees Celsius. An initiator was added to the mixture after it attains a steady state and the reaction was allowed to proceed for 5hrs. The mixture was cooled to 300 K and the colloidal dispersion was dialyzed for 7 days to remove any non-reacted reagents. The precipitate is then re-dispersed in water to obtain hydrogel colloidal solution. Three different PNIPAM hybrid dispersions solutions were prepared to find the optimal dispersion for photostable luminescent hybrid nanoparticle based bio-polymer for imaging. PNIPAM end capped with three functional groups; carboxyl group (PNIPAM (COOH)), thiol group (PNIPAM (SH)), and allylamine (PNIPAM (aa)) were used in this study. The PNIPAM-aa was synthesized from commercially available NIPA and the polymerization process was tailored to conjugate ZnO particles into the PNIPAM matrix.
For encapsulation ZnO nanoparticles in PNIPAM network, 2ml of 1.26wt% ZnO-water solution is homogenized at room temperature by stirring for a few minutes. Then 2ml of 2wt% of the hydrogel is added to 10 ml of water. The solution was continuously stirred for 5minutes to mon disperse the hydrogel particles and form a dilute hydrogel colloidal solution. The organic-inorganic hybrid of homogenized ZnO-water solution and dilute hydrogel colloidal solution are mixed and maintained for 24hrs. The resulting mixture is then centrifuged at 1000rpm for about 3hours and then re-dispersed in hydrogel. The chances of precipitation of ZnO from hydrogel solution can be reduced by controlling the centrifuge speed. ZnO dispersion stability appeared to be relatively higher in hydrogel than water. Also, the stability and mono dispersity of ZnO in hydrogel depends on the functional group present in the hydrogel.
Experimental techniques
The effects on continuous wave (CW) and time resolved (TR) photoluminescence (PL) of conjugating ZnO with PNIPAM gels are studied. Also zeta potential measurements were taken to quantify the stability of the hybrid-material in the solution. The excitation for CWPL was provided by a 15 mW 325nm line of a HeCd Ominchrome 56 series laser and the signal was collected with a Triax 320 spectrometer. Time resolved photoluminescence was measured using a Hamamatsu synchroscan streak camera with a resolution of 30 ps. The time-resolved photoluminescence measurements were performed using a 80MHz fem to second Ti: Sapphire mode-locked oscillator from Spectra Physics. The 700 nm laser output was doubled with a nonlinear β-Barium Borate (BBO) crystal to 350 nm. Furthermore stability testing of the three conjugates is performed. The zeta potential was measured by Coulter® DELSA™ 440 SX. The bio-imaging was performed using a multi-photon microscope.
Results and Discussion
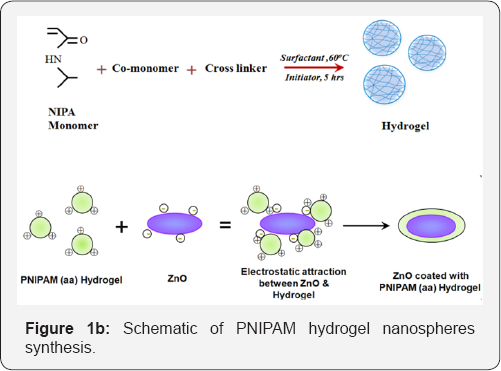
The average hydrodynamic radius and distribution of the ZnO-water dispersion, PNIPAM-aa colloidal particles and ZnO incorporated PNIPAM-aa hydrogel in dilute dispersions were measured using dynamic light scattering. The ZnO in water show an average hydrodynamic radius around 160nm whereas the PNIPAM-aa nanoparticles have a narrowly distribution with hydrodynamic radius ~ around 128nm. The ZnO based PNIPAM- aa hydrogel show an average hydrodynamic radius around 330350nm with a wide distribution confirming the dispersion of ZnO nanoparticles with in PNIPAM-aa colloidal solution. The adsorption of the PNIPAM hydrogel particles on the surface of ZnO nanoparticles results in an increase in the size of the ZnO- hydrogel composite compared to ZnO or PNIPAM particles. (Figure 1b) The effective size of the ZnO based PNIPAM-aa hydrogel network is comparable to the incident laser wavelength used for photoluminescence measurements (~325nm-350nm).
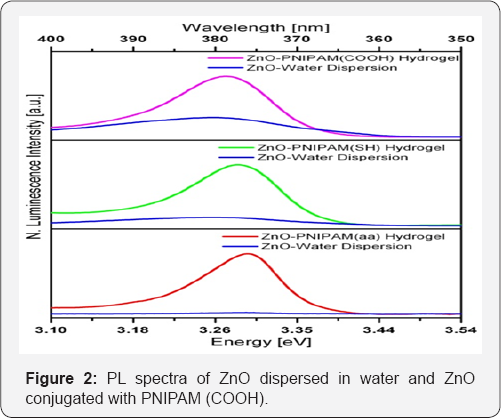
Figure 2 shows the CW photoluminescence spectra of each hybrid ZnO-PNIPAM solution along with the spectrum of the same concentration of ZnO nanoparticles dispersed in water. All ZnO-PNIPAM systems lead to an enhancement in PL intensity compared to just ZnO in water. The PNIPAM (aa) system provides the maximum enhancement. At least 10 times enhancement of the integrated PL intensity is observed from the ZnO within PNIPAM (aa) system compared to ZnO in water. All emission peaks have the same narrow full width half max (FWHM) of about 10 nm. This emission is narrower than common UV markers such as indole-tryptophan.
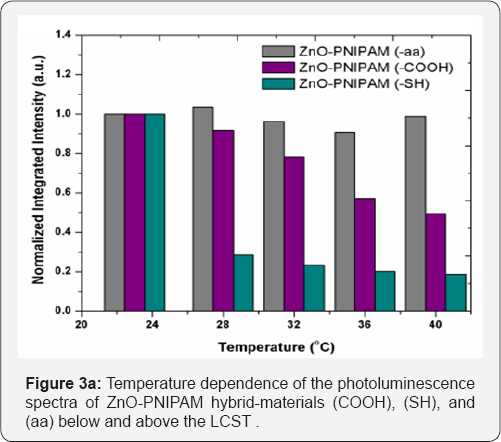
Figure 3a presents the temperature dependence of the ZnO- PNIPAM hybrid-materials. The temperature dependence of ZnO is normally negligible over a range of 15 K; however both the PNIPAM (COOH) and PNIPAM (SH) show a luminescence intensity drop of over 50 percent in this temperature range, suggesting an alteration in the emission of the ZnO due to hydrogels. The PNIPAM (aa) system however shows a minimal temperature dependence. Thus the PNIPAM (aa) does not alter the emission properties of ZnO the least not temperature dependent evolution. The histogram in Figure 3b summarizes the temperature dependent stability of ZnO in PNIPAM with various functional groups. The stability of PNIPAM-aa system is desirable since it retains more of the favorable emission properties of zinc oxide. Also a slight blue shift is observed for all samples as the temperature is likely due to the modification of the surface states of ZnO or change in the interface properties of ZnO-PNIPAM hybrid material system.
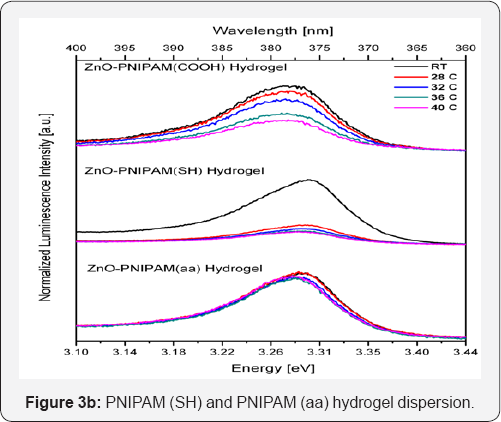
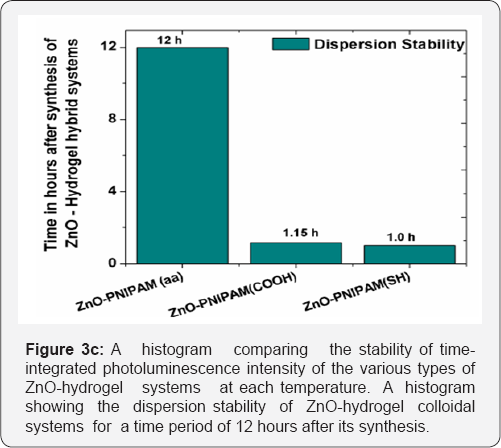
Stability testing was performed by finding the length of time during which the ZnO-PNIPAM hybrid systems stayed dispersed in aqueous solution and produced the same luminescence signal. Figure 3c shows the histogram depicting the stability of the ZnO nanoparticles in the PNIPAM solution with differing functional groups. The ZnO-PNIPAM (aa) proved to be the most stable and remained dispersed for two to three weeks, even after being heated. The PNIPAM (COOH) and PNIPAM (SH) hybrids remained dispersed for only about an hour. Thus the ZnO-PNIPAM (aa) is the ideal candidate marker for observing long biological processes. The emission is never photo bleached and remains stable and consistent throughout its lifetime. To gain a better understanding of the stability of PNIPAM (aa), zeta potential test was performed and the results are presented in Figure 4. The ZnO nanoparticles have a negative surface charge due to the synthesis of the nanoparticles under atmospheric conditions. Therefore a cross functional linker of the hydrogel with a positive ligand will be bound more strongly to the ZnO nanoparticles. It is expected that the ZnO nanoparticle dispersion will be more stable at a neutral pH and when the hydrogel nanoparticle network has a positive zeta potential.
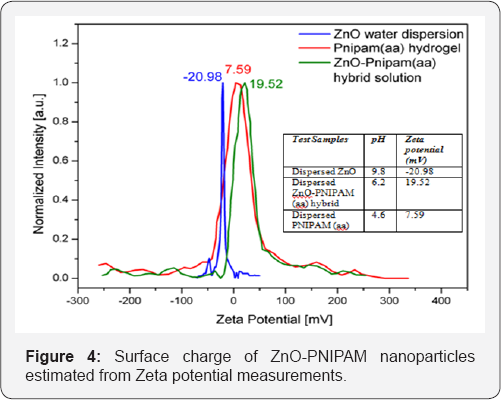
The ZnO dispersion with the PNIPAM-aa crosslinker has the greatest potential difference and is thus the most stable. Thus the incipient instability of the hybrid does not negatively impact experiments lasting days or weeks. The zeta potential for the PNIPAM-COOH is at -17mV and for the PNIPAM-SH it is -5mV. The surface of PNIPAM-COOH and PNIPAM-SH hydrogel nanospheres has a negative charge and is consequently less stable compared to PNIPAM-aa.
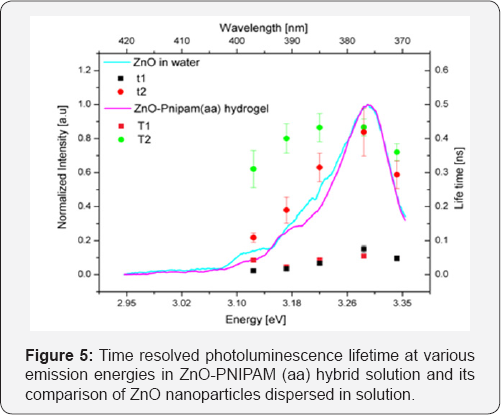
Lifetime studies were performed on the ZnO-PNIPAM (aa) hybrid nanospheres, the results are presented in Figure 5. The short component of the lifetime which is usually due to carriers recombining via non radiative channels at room temperature is not affected by the conjugation to the PNIPAM polymers. The longer lifetime component which is normally due to excitons is responsible for the majority of the emission. Figure 5 also shows that the radiative recombination process is also not significantly modified at ~ 3.28-3.30 eV. Therefore the enhancement in the PL emission is not due to the modification in the radiative or non- radiative recombination process. The presence of the hydrogel nanoparticle network with nanoparticle diameter of around 330-350 nm also scatters the incident photons at the pump laser wavelength. The scattering of the incident photon results in enhanced absorption of light by the ZnO nanoparticles, which is likely to be the source of enhanced emission. The exciton emission energy shift is also unaffected by the conjugation. There is a slight increase in the recombination lifetime of the shallow acceptors in ZnO between 380nm and 400nm. This increased lifetime reduces the probability of non-radiative transitions from the band-edge states to the defect level states and may contribute to the enhancement of the overall intensity.
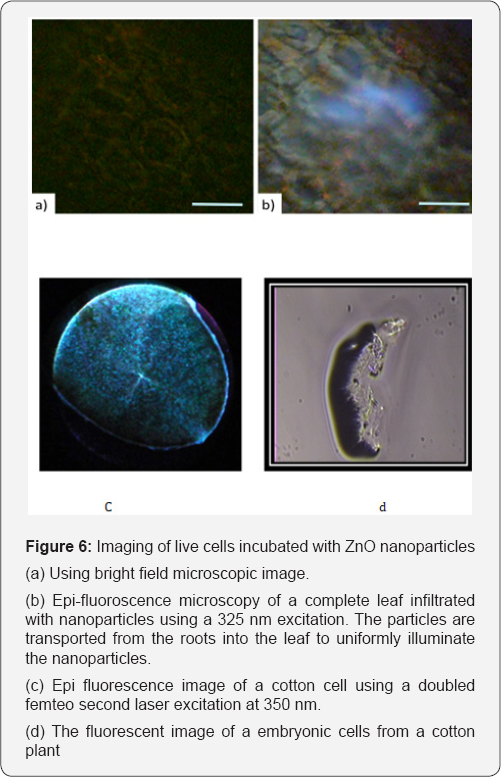
Conjugation of ZnO to functionalized amine PNIPAM hydrogels results in the enhancement of the PL signal in the bio compatible luminescence marker which may be engineered to react to different temperatures. This hybrid-material can also be used in drug delivery by swelling the polymer matrix with a drug and delivering it to selective areas by changing the temperature, thus shrinking the matrix and releasing the drug. It is always possible to track the material precisely using the luminescence of the ZnO particles which are non toxic and do not blink like many semiconductor quantum dots [14]. To demonstrate the compatibility of the ZnO nanoparticles for biological imaging, the nanoparticles were co-cultured with plant cells. The suspension cells of Arabidopsis thaliana and cotton were co-cultured with ZnO nanoparticles. The plant cells were incubated at 30 C and were subjected to sonication for about a week. The fluorescence image of the plant cells with and without the nanoparticles is shown in Figure 6a & b respectively. Figure 6c shows the fluorescent image of single plant leaf that was infiltrated with ZnO nanoparticles. The nanoparticles were transported through the roots and spread into the various regions of the plant leaf showing a wider area emission. Figure 6d shows the fluorescent image of a embryonic cells from a cotton plant.
Conclusion
The ZnO-PNIPAM (aa) system is found to be most stable in aqueous solution and to retain the favorable light emitting properties of ZnO. The PNIPAM (COOH) and PNIPAM (SH) hybrid material systems showed a luminescence enhancement, but were unstable. The luminescence of the ZnO nanoparticles can be significantly enhanced due to secondary scattering induced by the hydrogel nanoparticles. Using thus developed semiconductor - hydrogel hybrid meta-material will enable a researcher to track biological processes which take weeks without the concern of photo bleaching. The hydrogens unique ability to change its phase may be applied for drug delivery and allow for alteration of the biological system as it is observed by the ZnO in the hybrid material. Furthermore the hydrogel conjugation process is applicable to many luminescence markers, including quantum dots. The surface charged ZnO nanoparticles were also used as photo luminescent marker for imaging plant cells.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555572.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment