Use of Honey in Stabilization of ZnO Nanoparticles Synthesized via Hydrothermal Route and Assessment of their Antibacterial Activity and Cytotoxicity-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
Zinc oxide is a material having enormous application in the field of medicine, dentistry and cosmetics. It is an inorganic white colored solid insoluble in water and currently listed as Generally Listed As Safe material (GRAS) by food and drug administration. The nanoparticles of ZnO has found enormous application in the field of medicine and cosmetics [1,2]. At the same time, there is also raising issues on toxicity of ZnO nanoparticles and their subsequent applications in the field of medicine [3,4]. The organic stabilizers used in the synthesis of metal oxide nanoparticles in general and ZnO nanoparticles in particular are known to possess toxicity, hence are non-biocompatible and most of them are insoluble in water. The common stabilizing agents like hexadecylamine, troctylphosphine oxide, alkylpyridine, organic thiols, oleic acids etc are known to cause toxicity [5]. Colloidal ZnO has been mostly produced in aqueous or alcoholic solutions by direct reaction of zinc compounds with bases in the presence of appropriate stabilizers [6].
There are several methods to synthesize ZnO nanoparticles which include chemical vapor synthesis, Laser ablation, solvothermal, thermal decomposition and sol-gel method [7,8], which require use of costly and hazardous chemicals, use of enormous energy and associated environmental hazards. Hence the recent development in the field of nanotechnology research is more towards the green chemistry approaches, wherein the natural sources are used as stabilizing, reducing and capping agents in controlling the size and morphology of nanoparticles, specially the once whose biological applications are intended. In this respect, plant extracts of different parts of the plants in several organic solvents, microorganisms, carbohydrates and proteins have been used in the synthesis of nanoparticles [9-11].
Study on use of glucose and fructose as a stabilizing and reducing agents in synthesis of nanoparticles is gaining much popularity these days. There are reports that monosaccharides like glucose having linear chain and containing aldehyde groups are excellent reducing agents and it was also found earlier that fructose is capable of participating in the synthesis of metal nanoparticles of various morphologies [12]. As honey is a rich source of both these mentioned monosaccharides, in the current study, we used it as reducing and stabilizing agent. Furthermore, typical synthesis method used in synthesizing Zno nanoparticles is The hydrothermal technique.
It helps in processing monodispersed and highly homogeneous nanoparticles with precise control over the size and shape of nanoparticles. It also offers advantage of reduced energy consumption, better nucleation control, avoidance of environmental pollution etc [13]. ZnO is a common metal oxide used in restorative dentistry in the form of its chelate the Zinc eugenolate as a temporary and intermediate restorative material. The ZnO nanomaterial is known to possess antibacterial activity against many dental pathogens, especially the Streptococcus mutans which is the primary organism causing dental caries [14], hence in the current study, the antibacterial activity of the title material was assayed against S. mutans using spread plate technique [15].
As mentioned earlier, there is a raising question on toxicity profile of nanomaterials meant for food and medicinal applications, hence in the current study, we also evaluated the cytotoxicity of synthesized ZnO using Balb 3T3 mouse fibroblast cell lines [16], as the fibroblasts are the main component cells of periapical and periodontal tissues coming in contact with dental restorative materials.
Materials and Methods
Zinc acetate (99.9%) used in the current study was obtained from Alfa aeser, Sodium hydroxide from Loba-chemie and ultrapure water of resistivity 18.5 MΩ (Pure labs Q, ELGA). Pure honey was obtained from the nearby local market (Dabur India Pvt ltd). The Balb 3T3 cell lines used in the present study were obtained from National Centre for Cell Sciences (NCCS) Pune. Pure culture of S. mutans (MTCC 890) was obtained from Microbial Type Cell Culture and Gene Banking, Chandigarh. The Brain heart infusion broth and agar were procured from HIMEDIA Pvt Ltd.
Hydrothermal synthesis of ZnO nanoparticles
1M solution of Zinc acetate was dissolved in ultrapure water of resistivity 18.5 MΩ, which was stirred continuously at 60 °C. To this 0.1M sodium hydroxide was slowly added drop wise until the pH reached 9, at which a yellow colored precipitate was obtained. This was collected and washed several times with ultrapure water. Later the mixture was transferred to Teflon liner contained in stainless steel general purpose hydrothermal autoclave (SS 316). To this reaction mixture, 0.5 ml of honey was added as stabilizing and capping agent and treated under autogenous pressure at 180 °C for 12hr. Further, the autoclaves were allowed to cool and the obtained product was collected and washed several times with ultrapure water and finally with ethyl alcohol to remove the organic impurities and vacuum dried [17]. The obtained ZnO nanoparticles were sintered at 700 °C to improve crystallinity of the material.
Analytical methods
Hydrothermally synthesized ZnO nanoparticles were characterized using powder XRD, UV-Vis Spectroscopy, Field- emission Scanning Electron microscopy and Dynamic light scattering technique. Structure of ZnO nanostructures were analyzed using PXRD studies using Rigaku smart Lab-II, CuKa radiation. The optical absorption spectra were recorded using UV-Vis spectrophotometer (ELICO SA 165, India) in wavelength range of 200 to 800nm. External morphology of synthesized nanoparticles was determined using FESEM (Carl-Zeiss) and particle size was obtained by DLS using Microtrac Zeta analyzer.
Assessment of antibacterial activity using spread plate technique
1g of ZnO nanoparticles were dispensed in 1ml ultrapure water (1μg/μl) in a clean and dry Eppendorf's tube and sonicated for 45 min-1 hr to obtain a complete dispersion of ZnO. This was further serial diluted up to 0.125μg/μL. 25μL of fresh culture of S. mutans (in BHI broth) whose concentration was adjusted to 106 CFU/mL was added into each of the different concentration of ZnO dispersion. The tubes were sealed carefully and maintained at 37 °C with continuous shaking overnight.
Pen-Strep was used as negative control and overnight pure culture was maintained as positive control. After 24hr the Eppendorf tubes were removed from shaker. 20μL of each of the test sample was spread on the surface of BHI agar and incubated at 37 °C overnight. Next day the petri-plates were observed for growth or inhibition and a colony counting was performed using manual colony counter (Vision Microsystems).
Cytotoxicity studies of ZnO nanoparticles
Mouse fibroblast cell lines (Balb 3T3) used in the current study was obtained from NCCS, Pune and were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics (Himedia, 50μg/ml penicillin and 50μg/ml streptomycin) in 96 well-culture plates. Cultured cells were incubated at 37 °C in humidified atmosphere containing 5% CO2. Cells were regularly fed with Dulbecco's modified Eagles Medium (DMEM) and their growth was monitored by microscopic observations. Cells were regularly passaged by trypsinization with 0.1% trypsin in phosphate buffer saline (PBS).
CCK 8 allows sensitive colorimetric assays to determine cell viability in cell proliferation and cytotoxicity. The test was performed to evaluate cytotoxic effect of hydrothermally synthesized ZnO nanoparticles. The cells were seeded separately on 96-well microplates (Himedia) at density of 12,000 cells/ well and were allowed to grow for 24h in DMEM containing 10% FBS. The cells were incubated at 37 °C in a 99% humidified atmosphere containing 5% CO2.
1g of ZnO was dispensed into a 2ml capacity Eppendorf's tube to which 1ml of water ultra -pure was added and further the material was ultrasonicated for 45 min-1hr to obtain a complete dispersion. This dispersion was used for evaluation of cell viability of different selected cell lines. To check the cell viability, the cell lines were treated with ZnO (from 10μl to 1μl) and incubated for 24h. After 24h incubation, 10μl of CCK-8 (Sigma Aldrich) solution was added to each of the 96 well -plate and incubated at 37 °C in 99% humidified atmosphere containing 5% CO2 for 4 hr. Post incubation, absorbance was measured at 450nm using a microplate reader. Percentage cell growth was calculated from absorbance reading using equation

Results and Discussion
Analytical methods
Powder XRD: Purity of the phase of ZnO nanoparticles was measured by powder XRD analysis using Rigaku Mini Flux II (Japan) instrument using Cu as target material (1.5406 A). Crystal lattice indices of ZnO was confirmed by diffraction peaks observed at different 20 values. The average crystallite size was further determined using Scherrer formula

Where,
D=Average crystallite size
Θ=Bragg angle in degrees
β=Angular line width at half maximum intensity
λ= Wavelength of X ray used
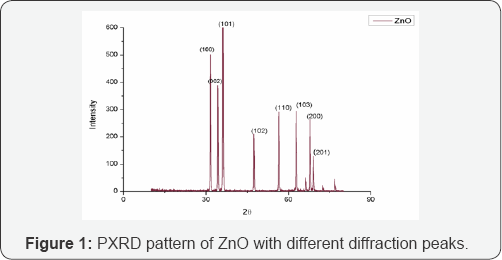
Figure 1 shows the PXRD pattern of hydrothermally synthesized ZnO nanoparticles. Diffraction peaks were observed at 2θ values of 31.7 °, 34. 3°, 36.1°, 47.4°, 56.5°, 62.9°, 66.2°, 67.8° and 68.9° corresponding to lattice planes (100), (002), (101), (100), (110), (103), (200), (112) and (201) respectively. The peaks have been attributed to hexagonal phase of ZnO (JCPDS file: 36-1451) [18]. All diffraction peaks are indexed according to the hexagonal phase of ZnO. The average crystallite size determined using Scherrer formula is 39nm.
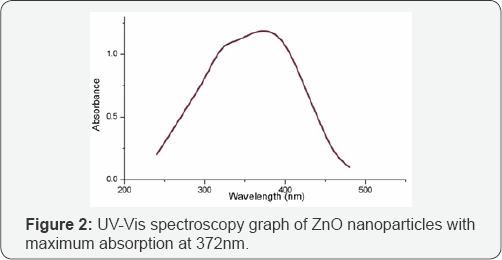
UV-Visible Spectroscopy: UV-Vis spectroscopy of synthesized ZnO nanoparticles was performed in wavelength range between 200-800nm (Elico SA 165, India) and absorption value was replotted using origin 8 software. Presence of maximum absorption at around 372nm confirms the presence of ZnO nanoparticles which is due to surface plasmon resonance of ZnO nanoparticles. A sharp peak was seen at 372nm correlating the formation of ZnO as supported by previous literature [19]. Figure 2 shows the UV-visible spectroscopy data of synthesized ZnO nanoparticles.
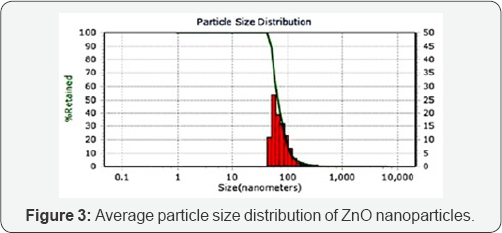
Particle size analysis using dynamic light scattering:Average size of the synthesized ZnO nanoparticles was measured using dynamic light scattering technique. In this method, Particle size can be determined by measuring the random changes in the intensity of light scattered from a suspension or solution. In the current study, results of the analysis show an average particle size distribution of 49nm with 95 percentiles of particles being at 48.9nm size range as seen in Figure 3.
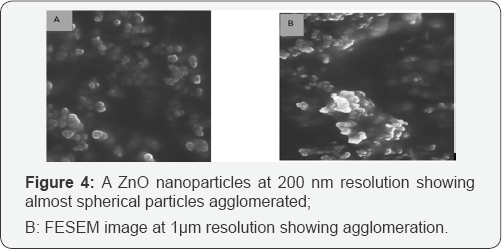
FESEM results: FE-SEM provides higher resolution in the whole range of accelerating voltages hence gives better picture quality and resolution compared to SEM. In the current study, FESEM of synthesized ZnO nanoparticles were performed using ULTRA 55, FESEM (Karl Zeiss). The particle dimensions and morphology of the as prepared ZnO nanoparticles were examined at different resolutions. Figure 4 shows the images of ZnO nanoparticles which look spherical in morphology with agglomeration, a common feature of ZnO nanoparticles [20].
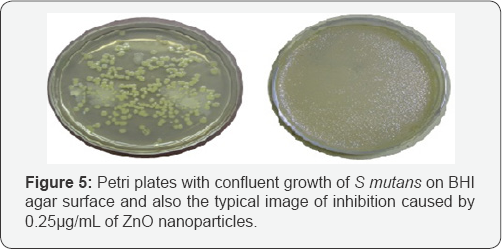
Antibacterial activity of ZnO nanoparticles against S mutans: After 24 hr incubation at 37 0C, the Petri plates were observed for growth or inhibition. The synthesized material showed inhibition of growth up to 0.25μg/μL, which is also its minimum inhibitory concentration. At lesser concentration, there was growth of round streptococcal colonies on the surface of BHI agar [21]. Positive control showed confluent growth of microbe and negative control showed complete inhibition. By this we can infer that as synthesized nanoparticles of ZnO possess antibacterial activity at concentrations as low as 0.25μg/ μL. Figure 5 shows the growth and inhibition of S mutans under the influence of ZnO nanoparticles.
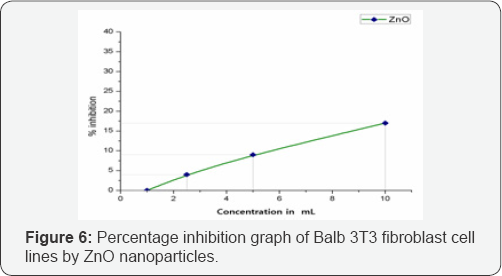
Cytotoxicity of ZnO on balb 3T3 cell lines: CCK 8 assay is water soluble tetrazolium salt based assay to screen the possible adherent cellular toxicity of various material. This assay measures cellular viability of fibroblast cells upon exposure to different concentrations of test substances. The graph remained almost linear as represented in Figure 6. At the highest tested concentration of 10μL the ZnO showed good growth promotion of 83% with only 17% inhibition in proliferation of cells. At the lower concentrations, the proliferative activity increased and touched base line at 1μL concentration. This shows that the synthesized material is not toxic to the selected cell lines at the tested concentrations. It has been proven earlier that large sized ZnO nanoparticles produces comparatively lower cytotoxicity, even though a base line for nanoparticle size determining the toxicity is not yet established, but in typical experiment performed by current authors the nanoparticles were nearly 49nm which proved to be less toxic [22].
Conclusion
From the current study, we conclude that honey was successfully used as stabilizing and reducing agent in hydrothermal synthesis of ZnO nanoparticles. Thus synthesized material had good antibacterial activity against S. mutans, the primary organism causing dental caries. Also, the material remained negligibly toxic to Balb 3T3 mouse cell lines which declined with reduction in concentration. Hence, the title material can be considered for further animal trials to subsequently develop a product with potential antimicrobial application in dentistry with no toxic effects.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555585.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment