Nanostructured Lipid Carrier (NLC): A Promising Drug Delivery System-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
Oral route is the most preferred and acceptable way of drug administration because of low cost and ease of administration which leads to high patient compliance. But lipophilic drugs show low oral bioavailability due to various reasons like poor aqueous solubility, high first pass metabolism. Lipid Matrices have been used for a long time as a carrier for lipophilic drugs to enhance their oral bioavailability. Various lipid particulate carriers have been developed and studied for many years like Lipid emulsions (LE), liposomes, Solid lipid nanoparticles (SLN) and nanostructured lipid carrier (NLC). Eldem et al. [1] had optimized the production of lipid micro pellets. The first generation of lipid nanopellets for oral administration was prepared by Speiser et al. [2]. This was followed by the second generation of lipid matrices involving the production of SLN by high pressure homogenization (HPH) [3].
Gasco developed microemulsion technique for the production of solid lipid microspheres in 1993 [4]. SLN had various advantages over lipid emulsion such as being solid at room temperature, ease of dosing and handling. Mader and Mehnert showed various limitations of SLN such as-poor drug loading, high water content and drug expulsion during storage due to the formation of more stable lipid structure.
To overcome the above problems, a third generation of lipid matrix was designed as Nanostructured Lipid Carrier (NLC) [5]. The paper describes the different types of NLC including production of highly concentrated particle dispersions, mechanism of absorption and their application as drug delivery system.
NLC & types
NLC is a blend of mixture of solid lipids along with spatially incompatible liquid lipids. It remains solid at room temperature. It has various advantages similar to SLN such as use of bio-compatible lipids, controlled release of drug from the carrier, feasible to produce on large scale using the existing machinery at economical cost, avoids first pass metabolism by lymphatic transport and provides protection to drug moiety from biochemical degradation. It also overcomes the disadvantages of SLN such as NLC have better drug loading due to the use of liquid lipid, avoids drug expulsion during storage for long period, lipids can be used in higher ratio(up to 95%) when compared to SLN where above 30% it forms bicoherent systems.
NLC can be classified into three different types based on the nano-structure (Figure 1), composition and ratios of solid and liquid lipids-
a. The imperfect type
b. The multiple O/F/W type
c. The amorphous type
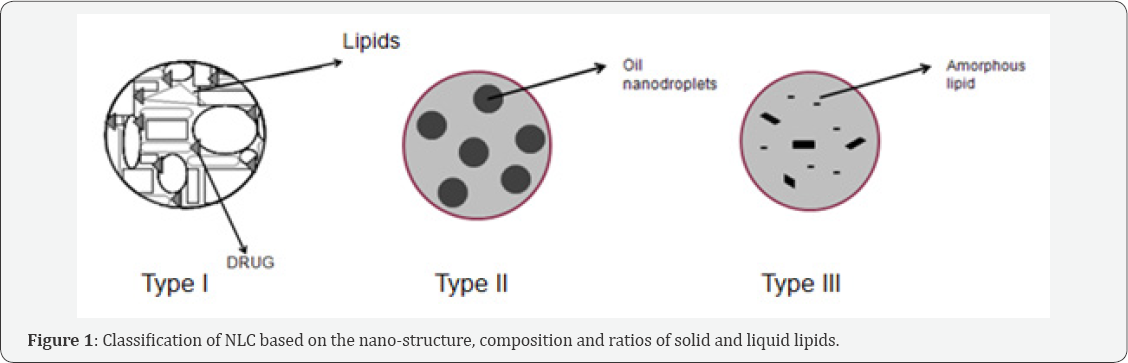
The imperfect type: This is produced by mixing chemically different solid and liquid lipids which gives rise to imperfections and hence enhanced drug loading.
The multiple O/F/W type: This contains oil nanocompartments encapsulated with solid lipid. The drug is loaded/ dissolved in the oil compartments. It was prepared by lipid- lipid precipitation technique.
The amorphous type: This is prepared by controlled mixture of special types of solid and liquid lipids (eg, Isopropylmyristate) such that the NLC obtained are in amorphous state.
Composition of NLC (Table 1)
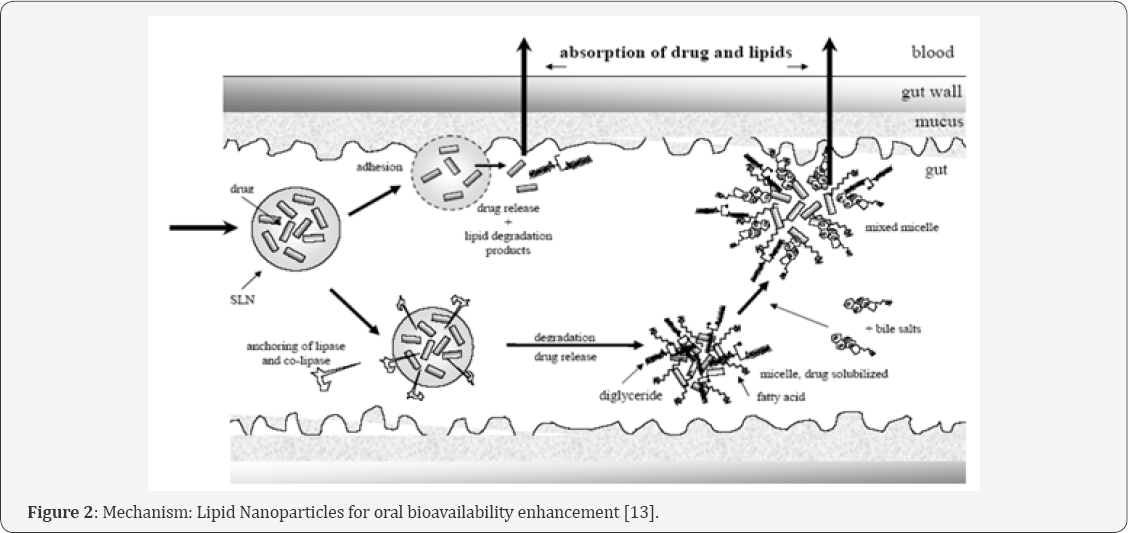
Different mechanisms of the NLC for improved bio availability
Direct uptake: By GI tract i.e., by Intestinal lymphatic transport. Due to the lipophilic nature and use of long chain triglycerides, the NLC may stimulate the chylomicron formation, which follows the transcellular route of absorption. Highly lipophilic compounds are transported by intestinal lymphatic system which avoids hepatic first pass metabolism. The compounds are transported in association with the triglyceride (TG) lipid core of the intestinal lipoproteins hence lipids are required to for lipoprotein formation.TG hydrolysis begins in the GIT by lingual lipase & gastric lipase to form a crude TG emulsion, which is then emptied into duodenum. This crude emulsion stimulates the production of bile salts, biliary lipids and pancreatic juice. TG emulsion is stabilized by the adsorption of biliary lipids onto its surface. Pancreatic lipase acts at the surface of emulsified TG droplets to form monoglyceride (MG) and fatty acid (FA).
The long chain FA and MG are absorbed by the enterocyte. The absorbed FA is re-esterified by mono-acylglycerol pathway. These are then processed by number of organelles where they are arranged to become the lipid core of chylomicron. The formed chylomicrons are stabilized by the addition of phospholipids & apolipoproteins [6]. These stabilized lipoproteins are then secreted into lamina propria and mesenteric lymphatic [7].
Muco-adhesion: The lipid nanoparticles adhere to the mucus thereby increasing the residence time and hence there is increased release of drug from the carrier [8].
Mixed micelle formation: The lipids which are used in NLC are similar to dietary lipids and thus induce bile secretion in small intestine. The lipids which undergo degradation upon the action of enzymes to form lipid digestion products and are mixed with bile to form mixed micelle. This phenomena causes increased solubilisation of drug and hence facilitate its transport across the membrane [9].
Increased permeability: Surfactants alter the intestinal permeability by various mechanisms. For eg. Poloxamer is known to deform the cell membrane and open the tight junction of intestinal epithelial cell facilitating paracellular transport of NLCs [10]. It also inhibits P-gp efflux and increases NLC transport across the intestinal mucosa. These also provide the required steric stabilization [11].
Inhibits drug degradation: Some drugs are unstable in the harsh GI environment. NLC offer the advantage of protection of drug by the lipids from chemical & enzymatic degradation, thereby delaying in vivo metabolism (Figure 2) [12].
Preparation Methods
High pressure homogenizer
This is the most common technique used for the production of NLC. It has advantages over other techniques which include no use of organic solvents, feasibility of large scale production. There are two methods which can be followed, hot homogenization technique and cold homogenization technique [13-15].
In both the methods, the first step involves dissolution of drug in melted lipid mixture (LM) (solid+liquid) at 5-10 °C above the melting point of lipid.
In hot homogenization method, the above melt is dispersed in hot aqueous surfactant solution by stirring to obtain emulsion and was homogenized in HPH at same temperature to obtain hot nano-emulsion. Further cooled to room temperature to obtain NLC.
In cold homogenization method, the above melt (step-1) was solidified and ground to obtain lipid micro particles. These were dispersed in cold aqueous surfactant solution to obtain pre-suspension and homogenized at reduced/RT to obtain NLC. This method is useful for hydrophilic drugs.
Micro-emulsion technique
The LM, lipophilic surfactant were melted at 5-10 °C above the melting point of lipid to which the drug was added for complete solubilization. The aqueous surfactant solution at same temperature was added to lipid melt under stirring. A clear micro-emulsion was obtained when the components were mixed in correct ratio. It was then dispersed into ice-cold water under continuous mild stirring to form NLC. Here, the small size of the particles is due to precipitation and not mechanically induced by stirring.
Solvent- diffusion method
It consists of two different phases; organic phase consists of the LM, lipophilic surfactant and drug which are dissolved in organic solvents at elevated temperature. The resultant organic solution was quickly dispersed into the aqueous surfactant solution at room temperature (25 °C) under high mechanical agitation for a period of time to obtain NLC. The obtained dispersion can be placed in vacuum desiccators for 24h to evaporate the residual organic solvent [16].
Melt emulsification technique
The lipid mix, surfactant and drug were melted and dispersed in hot aqueous surfactant solution at same temperature using mild stirring to form primary emulsion. The warm primary emulsion was subjected to lab ultrasonic cell pulverizer to obtain nano-emulsion whish was rapidly cooled by immersing beaker in ice-cold water and stirred to obtain uniform NLC dispersion [17].
Solvent evaporation technique
This technique involves the addition of LM and drug in organic solvent which is then emulsified in an aqueous phase. After evaporation of the solvent the lipid precipitates forming NLC.
Characterization
Drug entrapment efficiency (Ee) and drug loading (DL):The obtained dispersion of NLC was centrifuged at high rpm (10000-20000) for a period of 15-20min. The rpm was selected based on the particle size and hence lesser the size, higher the rpm required to centrifuge. The supernatant was analyzed by suitable HPLC method to determine the amount of drug in it.
The drug entrapment efficiency (Ee) and drug loading (DL) were calculated as follows:
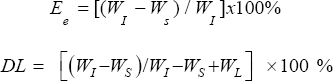
Where,
WI = weight of drug added initially
Ws = weight of drug in supernatant
WL = weight of lipid mixture added
Ee can also be determined by suitable analysis of the obtained NLC and calculated as follows:
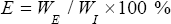
Where WE = weight of the drug entrapped in NLC.
Particle size (PS) and zeta potential (ZP): Particle Size an Zeta Potential of NLC were measured by Photon correlation Spectroscopy (PCS) using Malvern Zetasizer which works on Mie theory. All the size & ZP measurements were carried out at 25 °C after appropriate dilutions. The ZP determination was based on particle electrophoretic mobility in aqueous medium. The ZP characterizes the surface charge and gives the information about long term stability. At higher ZP the particle aggregation is less likely due to electric repulsion. Generally the ZP of dispersion should be either less than -30mV or greater than +30mV for the NLC to be stable [18].
Poly dispersity index: It is determined by the equatio PDI= (D (0.9)-D(0.1))/D(0.5)
Where,
D (0.9) = PS immediately above 90% of sample
D (0.5) = PS immediately above 50% of sample
D (0.1) = PS immediately above 10% of sample
The PI is the measure of size distribution of the NLC. PI value ranges from 0.0000 to 1.0000 i.e., monodisperse to very broad particle size distribution.
External morphological studies: These studies are used to visualize the particle morphology. In SEM, the samples are suitably diluted and spread on sample holder & dried using vacuum. These are subsequently coated with gold and examined by SEM. In TEM the diluted sample is placed on carbon coated grids and dried to form thin film. It is then stained with phosphotungstic acid, dried and probed by TEM.
Crystallinity studies
DSC Studies
Thermal analysis of the obtained NLC formulation was carried out using DSC to determine the recrystallization Index (RI). Accurately weight sample (3-5mg) was places in aluminum pan and sealed with lid. An empty Al pan was used as reference. Samples were heated at suitable heating rate of 5-10 °C/min over a range of temperature. The RI was calculated as

Where,
ΔHNLC = enthalpy of fusion of NLC NLC
ΔHlipid = enthalpy of fusion of Lipid.
The melting point depression in the sample may also be attributed to the reduced size of nano particles.
Wide angle XRD
The crystallinity of NLCs was further determined by Wide Angled XRD using x-ray diffractometer. It is used to determine the geometric scattering of x-ray from crystal plane to assess the degree of crystallinity. Diffraction pattern with a relative sharp peak indicates the crystalline nature. Diffused x-ray scattering indicate low molecular order and crystallinity [19,20]. It is used to complement the results obtained from DSC.
Application of NLC in drug delivery
Basically, the NLC can be applied for all applications described for SLN. Areas of highest interest are oral and topical delivery, that means regarding short time two-market and lowest regulatory hurdles. Zhang et al. [21] has described the characterization and evaluation of NLC as vehicle for oral delivery of Etoposide. Pharmacokinetic studies have revealed prolong t-max and improved relative bioavailability of drug- NLC in comparison to drug suspension in rats after oral administration. Again a similar type of study by Thatipamula et al. [22] encapsulating Domperidone in both SLN & NLC as oral carrier showed that NLC had higher Ee and consistent faster release when compared to SLN. The formulation was stable after 40days of storage.
The study by Chen et al. [23] on effect of lipophilic emulsifiers on the oral administration of Lovastatin from NLC showed significant improvement in bioavailability when compared to free solution. Myverol-NLC was more stable in gastric environment when compared to SPC-NLC indicating the role of emulsifier. Jain et al. [16] described the development of NLC for oral hypoglycemic agents. The effect of surfactant blend on NLC was studied revealing that the formulation containing lecithin, poloxamer 188 and sodium taurocholate exhibited better and good release profile. The study by Zhuang et al. [24] on preparation and characterization of Vinpocetine loaded NLC upon In-vivo PK study revealed improved relative bioavailability of NLC up to 300%, when compared to the suspension in wistar rats on oral administration. Tiwari et al. [9] Compared NLC against SLN for Simvastatin, PK study revealed that the NLC has higher relative bioavailability when compared to both suspension & SLN. Full range of excipients is available being of accepted state (e.g. all lipids and surfactants used in creams, tablets, pellets and capsules). Of special interest are life-time extensions of drugs (e.g. prednicarbatein topicals, cyclosporine for oral delivery).
Due to the special character of NLC, there is no or little possible infringement regarding other patents. There are many patents on emulsions, micro-emulsions and liposomes, but only a limited number on lipid nanoparticles made from solid lipids, especially made from mixtures of solid and liquid lipids. There are also some niche applications, for example ocular delivery of nanoparticles to prolong the retention time. Many papers describe the prolonged retention of drugs in the eye using polymeric nanoparticles; however, up to now no product is on the market due to various reasons (e.g. toxicity problem of nonaccepted polymer poly alkyl-cyanoacrylate).
SLN showed an increased retention time in the eye, it would be even more beneficial to use NLC with improved drug accommodation properties. Andrade et al. [25] design and evaluate the potential of a topical delivery system for ocular administration of voriconazole, based on cationic nanostructured lipid carriers (NLCs). Similarly Souto et al. [26] studied major challenges in ocular drug therapy. They reviewed the promising strategies of colloidal carrier systems characterized by a submicron-meter size. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) represent promising alternatives to conventional and very popular ocular carrier systems, such as the nanoemulsions, liposomes, and polymeric nanoparticles. The reviewer's particular emphasis to the clinical choices currently available, while examining the most recent drugs that have been approved.
Similar to nano suspensions nebulised aqueous NLC dispersions could be exploited for drug delivery to the lungs. Wang et al. [27] engineer a nano-drug delivery system for co- encapsulating PTX and DOX. This system was expected to resolve the multidrug resistance caused by single drug, and the dualdrug-loaded nanostructured lipid carriers were also planned to specifically target the cancer cells without obvious influence on normal cells and tissues. The PTX-DOX NLC constructed in this research offers an effective strategy for targeted combinational lung cancer therapy. With any simple, mechanical nebuliser these dispersions could be administered. Identical to SLN, a basic advantage of NLC is that a novel encapsulation technology can be combined with a traditional dosage form well known to the patient, e.g. tablet or pellet. The aqueous dispersions can be used as granulation fluid for tablet production or wetting agent for extrusion of pellets.
Conclusion
Hence, based on the above review revealed that NLC is considered being the smarter, latest generation of lipid nanoparticles possessing improved properties for drug loading, modulation of release profile and stable drug incorporation during storage. Due to the various advantages of NLC it can be easily used as a carrier for drug via oral route of administration. Over the past decade, NLC formulation has undergone a continual improvement in the biomedical field. Both amendments played a major role in the application of NLCs and achieving successful outcomes. NLCs have a number of potential industrial applications, mainly due to the many advantages, which have induced the publication of many patents for different applications. However, many of them derive from basic research, and more industrially driven studies are needed. The industrial applications for the vectorization of therapeutically relevant molecules, as well as biotechnological products such as proteins and genetic material.
The production technology for highly concentrated lipid particle dispersions—developed in parallel to the NLC technology being applicable to both NLC and SLN-eases the transform of aqueous dispersions to solid products, e.g. tablets, pellets, capsules, but also powders for reconstitution. A lot of preclinical and clinical examples described in the review through clearly illustrate the promising application of the nanoformulation for the improving the existing technological drawback for the pharmaceutical market and taking into account an increasing number of patented NLC-based formulations. These advantages are thus overcoming previous existing problems. It is also feasible to produce on large scale thus making it one of the promising deliveries, which can be seen in near future in the pharmaceutical market.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555575.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment