Enhanced Nanocurcumin uptake using Electrical Pulses in Regulating MCF-7 Tumor Cell Growth and Apoptosis-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
Worldwide, breast cancer (BC) is the most common cancer diagnosed in women, with approximately 1.3 million new cases per year [1]. With an estimated 232,240 new cases of invasive breast cancer (Stages I-IV), and an estimated 64,640 new cases of ductal carcinoma in-situ, Stage 0, occurring in the USA, one woman dies every 13minutes from breast cancer [1,2]. Conventional breast cancer treatments like chemotherapy, radiation therapy, and surgery have drawbacks and may not be applicable to all patients [2,3]. There have been many drugs developed to treat cancer overall but they have exhibited limited success, due to inefficient delivery methods that allow molecules to cross the hydrophilic/hydrophobic lipid bilayers of cellular plasma membranes [4]. To overcome this limitation, electrochemotherapy (ECT) has been a process gaining momentum. ECT is the local application of electrical pulses directly to tumor tissue to render the cells permeable to impermeant drugs such as bleomycin [2,4-6].
Once bleomycin has been introduced into the cell, there occurs a most potent cytotoxic effect. However, clinical experience to date, while encouraging, is still not optimal for drug treatment using ECT with bleomycin [5]. Although, ECT with bleomycin is a promising therapy, it is of practical interest to find a gentler drug with high efficacy. One bioactive constituent is curcumin. Curcumin is the yellow pigment of the spice, turmeric, which has a history of over 6,000 years of medicinal utilization [7] and is derived from the rhizomes of Curcuma longa, a herbaceous perennial plant of the ginger family [8-10]. Turmeric is a naturally occurring polyphenolic phytochemical that exhibits antiseptic, anti-inflammatory, antioxidant, chemopreventive, and chemotherapeutic properties [9].
There has been evidence to show its effective cytotoxic, antiproliferative, and apoptotic activity in-vitro, as well as tumorigenesis suppression within rat models, making curcumin attractive as a chemotherapeutic agent [11]. The drawback is that its bioavailability in animals and humans remains low which relates to its inadequate absorption into tissues and its rapid metabolism. To illustrate, curcumin bioavailability from standardized Curcumin extract was poor on colorectal cancer patients in some clinical studies. To increase curcumin's bioavailability, it is possible to encapsulate using nanoparticles and using electrical pulses to locoregionally uptake this compound as recently shown in our lab, using the human breast cancer cell line, MCF-7. In this study, we have utilized curcumin- loaded nanoparticles (nanocurcumin) to assess the apoptotic activity within the MCF-7 cells and show that the curcumin increases programmed cell death with this cancer cell type.
Materials & Methods
MCF-7 cancer cell line
Estrogen receptor positive MCF-7 (human, Caucasian, breast adenocarcinoma) cells were used. Cells were cultured in 90% MEM media +10% FBS (ATTC, Manassas, VA) and 1% Penicillin/ Streptomysin (Invitrogen, Carlsbad, CA). Cells were grown in an incubator at 37 °C at 95% humidity and 5% CO2.
Electroporation of MCF-7 cells
Cells were dissociated from flask by treatment with 0.25% trypsin/EDTA solution (Invitrogen, Carlsbad, CA). Cells were counted using a cellometer and resuspended in MEM media to a concentration of 250,000 cells per 0.4cm cuvette tubes. A BTX 830 square wave electroporator (Genetronics, San Diego, CA) along with 0.4cm cuvettes were used for electroporation. The parameters for electroporation were 1200V/cm, 100μs, 8 pulses, at one-second interval. Cells were pulsed in media without 10% FBS or in RPMI media. Cells were removed after pulsing from the cuvettes and seeded in 6 well plates. Cells were then incubated at 37 °C in a 5% CO2 atmosphere at 95% humidity over a period of 72hours.
Experimental design
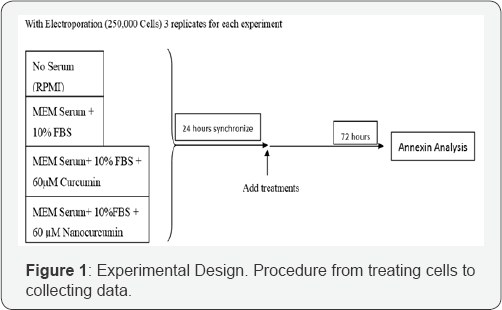
Nanocurcumin synthesis
Nanocurcumin generation began by utilizing 250g water- soluble glycol chitosan (cat. #G7753-500MG, Sigma-Aldrich, St.Louis, MO) conjugated to 75mg 5-Cholanic acid (cat. #C7628- 1G, Sigma-Aldrich) to form self-assembled hydrophobically- modified glycol chitosan (HGC) nanoparticles in an aqueous solution according to previously published data [12-14]. Next, monodispersed super paramagnetic iron oxide nanoparticles (SPION) were made by mixing 0.178 g of iron (III) oxide (FeO(OH), cat. #371254-50G, Sigma-Aldrich), 2.26g oleic acid (cat. #364525-25ML, Sigma-Aldrich), and 5g of 1-octadecene (cat. #O806-25ML, Sigma-Aldrich) followed by heating the mixture to 320°C with constant stirring under a nitrogen atmosphere.
The resultant SPION were further purified, processed, and ultimately physically loaded into 12mg HGC nanoparticles by using a probe-type sonicator at 4 °C. The HGC nanoparticles were further processed with curcumin (cat. C7727-500MG, Sigma- Aldrich) such that the curcumin was solubilized in ethanol followed by attachment to the HGC nanoparticles via adsorption onto the iron oxide surfaces of the nanoparticles.
Effect of nanocurcumin on MCF-7 cells
The effect of nanocurcumin, without electroporation, on MCF-7 cell growth and viability was determined. The effect of cell growth after 72hours were lower when compared to that of curcumin and without 10% FBS serum treatment. Control cells were treated with MEM media and 10% FBS. Cells were seeded in 6 well plates in 3 replicates at 37 0C for 72hours.
Effect of electroporation coupled with nanocurcumin on MCF-7 cells
The effect of nanocurcumin, with electroporation on MCF- 7 cell growth and viability was determined. The effect of cell growth after 72hours were more effective when coupled with electroporation at 1200V/cm. There were higher late apoptosis than dead cells or live cells.
MCF-7 cell viability and growth assay and MUSE flow cytometer
Following electroporation and incubation for 72hours, cell count for live and dead cells (viability), and total numbers of cells (growth) were done for each electroporation treatment. Three replicates for each treatment were counted using the MUSE flow cytometer. Annexin V and Dead Cell Assay Kit (EMD Millipore, Billerica, MA) was utilized, 100μL of Annexin V and Dead Cell Reagent was added to a 1.5mL conical tube, 100|iL of cells in suspension was added to each tube, and incubation of 20minutes at room temperature before counting. The average was taken from three replicates in each experiment for each treatment.
Statistical analysis
Results are expressed as mean ± SEM. Analyses were performed using one way and repeated measures of ANOVA.
Results and Analysis
Effect of electroporation on MCF-7 cell growth and viability
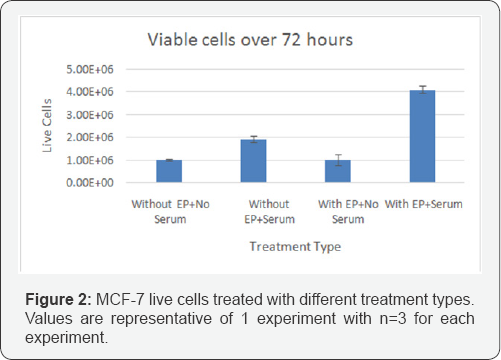
Figure 2 shows the viable cell counts over 72hours for various samples. In control samples, there is some growth observed of MCF-7 cells in the presence of serum, while there is no exposure to EP. Interestingly, the EP treatment did not result in a reduction in cell numbers when there was no serum present. This indicates the EP did not cause irreversible damage and cell death. When cells were treated with EP and serum, there was a significant 2 fold increase in cell numbers. This increase suggests that EP lead to transient openings in cell membranes that allowed serum components access to the inner cell and cytoplasm, thus promoting cell growth.
Effect of nanocurcumin on MCF-7 cell growth and viability (Without Electroporation)
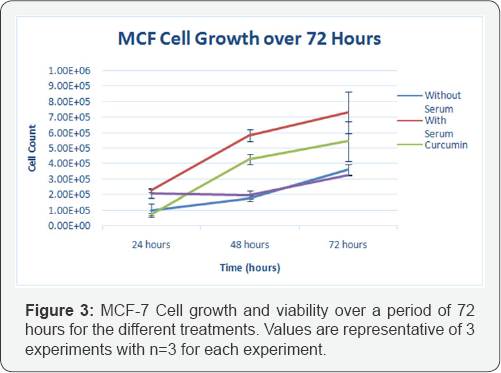
Figure 3 shows MCF-7 Cell growth and viability over a period of 72hours for the different treatments, using nanocurcumin and no electroporation. A sustained growth and doubling of cell numbers occurred in MCF-7 control cells treated with serum. It takes 48 hours after addition of the 60|im nanocurcumin to effect the cells. At 24hours, there were less cell count in the curcumin treatment which was expected as curcumin induces immediate apoptotic event after addition of 60|im curcumin. At 48 and 72hours, there was an increase in cell count for MCF-7 cells with curcumin, whereas the MCF-7 cells with nanocurcumin stayed relatively the same number of cell count throughout the 72hour period.
Effect of electroporation on MCF-7 cell growth and viability)
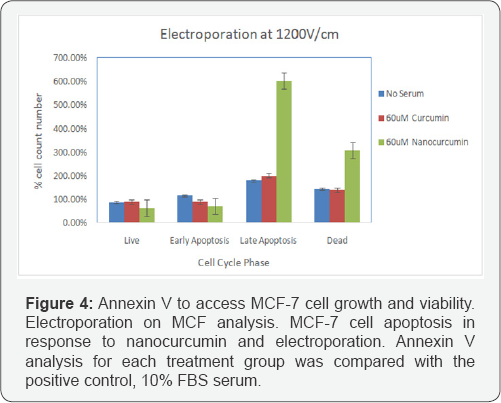
Figure 4 shows the Annexin assay results on cell growth. The cells were electroporated using eight, 1200V/cm, 1000|is pulses at one second interval. At the 72 hour time point, curcumin did not have a induce apoptosis in MCF-7 cells. However, in the present of EP the treatment of cells with nanocurcumin resulted in a significantly higher percentage of dead MCF-7 cells, and cells that were in late apoptosis. This could be the result of enhanced entry of nanocurcumin into the cells and due to the increased half life of curcumin that has been encapsulated.
Discussion & Conclusion
Use of nanoparticles for cancer therapy has been gaining momentum [15-18]. Rodzinski et al. [16] reported about use to magnetoelectric nanopartilces for targeted and controlled anticancer drug delivery. They studied using both in-vitro and in-vivo models and found that the nanoparticles were localized in the tumor sites. Sun et al. [17] discuss in their work on engineered nanoparticles for drug delivery in cancer therapy. In this review, they discuss the critical need for nanoparticle carriers for the delivery of various types of cancer therapeutic agents, such as hydrophilic, hydrophobic, and highly charged, and their design, synthesis and fabrication. They also indicate some general challenges in targeted delivery of nanoparticle carriers, such as endocytosis, intracellular transport, biodistribution, biocompatibility, and bioavailability, under both in-vitro and in-vivo conditions. Huang et al. [18] used gold nanaopartciles using electroporation for enhanced polyplex delivery of DNA and siRNA, into mammalian cells. Both anchor cells (NIH 3T3) and suspension cells (K562) were studied and found 1.5 to 2x improved transfection efficiency with no significant increae of toxicity compared to delivery of plasmid only using electroporation alone. They also used BTX ECM 830 electroporator that was used in our study, with 2mm electrode gap cuvettes. The pulse parameters applied were: 625V/cm, 10ms, single unipolar pulse.
Encapsulating curcumin using polymeric nanopartciles, known as nanocurcumin and its efficacy on cancer cells was also studied by Bisht, et al. [19], on pancreatic cells. In this study on eight pancreatic cell lines, they studied the effect of polymeric nanoparticle-encapsulated curcumin. The cell lines were exposed to a 20x range of void polymeric nanoparticles (93-1852μg/ mL). The size of the nanoparticles was 50nm. Viability study after 72hours indicated no cytotoxicity to the cell lines. Unlike normal curcumin, nanocurcumin readily dispersed in aqueous media and it demonstrated comparable in-vitro therapeutic efficacy compared to normal curcumin. Further, nanocurcumin's mechanisms of action on pancreatic ells are comparable to that of normal curcumin, including induction of cellular apoptosis, blockade of nuclear factor kappa B (NFкB) activation, and down regulation of steady state levels of multiple pro-inflammatory cytokines (IL-6, IL-8, and TNFα).
Our results indicated that use of nanocurcumin, uploaded using electrical pulses has the potential to treat various cancers that are refractory to current standard or cure. Considering that there were 14.1million new cancer cases and 8.2million deaths in 2012 and it is estimated that both the number of new cases and deaths will increase to over 20million and 10million respectively, by 2030, there is a critical need for alternate therapies. Electrical-pulse-mediated chemotherapy using nanocurcumin is a promising candidate for further studies to transfer this technique from lab to the clinic.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555574.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment