ZnO Nanostructure Deposition on Si Substrates by Chemical Vapor Deposition and its Antibacterial effect on Escherichia coli-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
The nanostructures material with specific properties dependent on their size and geometrical shape has created an interesting research environment for scientist in different fields of application. In particular, noble metals have found applications in optics [1,2], electronic [3,4], biomedicine and antibacterial activity [5-7], and quantum size domain [8]. ZnO nanostructures, such as ZnO nanoparticles, provide a large surface-area-to-volume ratio and a high fraction of surface atoms [9], which enhance the antimicrobial activity of ZnO, even at low concentrations [10,11]. ZnO has been generally found to be an n-type semiconductor with a band gap of about 3.3 eV, i.e. absorption begins near the UV A area. ZnO thin films have a great variety of potential applications such as light emitting diodes, laser diodes, solar cells, liquid crystal displays, thin film transistors, and gas sensors [12,13]. ZnO is also applicable to air and water purification and waste remediation due to its photoactive and photo catalytic properties. In addition ZnO is antibacterial and has already been studied for several decades in antibacterial tests, and orthopedic and dental clinical treatments [13-15].
In recent years, the synthesis of ZnO nanostructures has received considerable attention because of their ease of growth and potential applications in optoelectronic devices, including light emitting diodes, laser diodes, and nano-piezotronics [16,17]. Chemical vapor deposition (CVD) is very attractive as a simple cost-effective means of preparing ZnO thin film and nanostructures. Normally, pure zinc powder is used as the Zn source to prepared CVD grown ZnO nanostructures. In this work, we have used this technique and produced ZnO nanostructures and investigated their antibacterial properties as well as structural characteristics.
Experimental Details
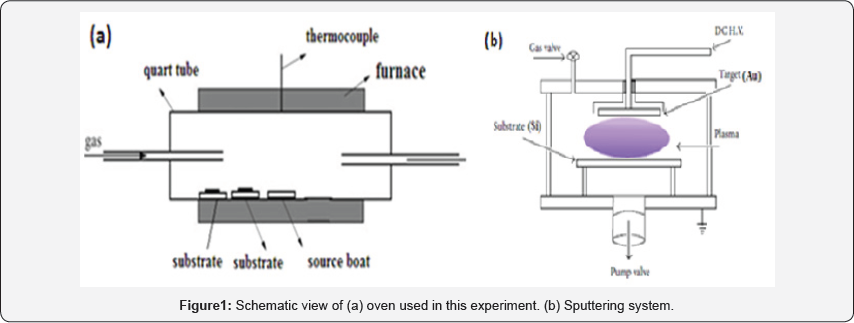
Our experiment performs in two step: I) Deposition of Au catalyst on Si substrate (Au/Si), II) Deposition of ZnO nanostructure on Au/Si (ZnO/Au/Si). The experimental system for ZnO deposition is as illustrated in (Figure 1a). Zinc powder was used as the evaporating source. The source was contained in an alumina boat placed at the centre of a quartz tube which was inserted in a horizontal tube furnace. The temperature of the system is controlled by a thermocouple inserted in the middle of furnace. The reacting gas (Ar/O2) was introduced into the system by gas injector at different locations to achieve the best uniformity over a given substrate size. At the beginning of the experiment argon was introduced into the system for an hour to purge the system. Then the furnace was heated at an approximate rate of 80oC/min to the set temperature under the same Ar flow rate. Then the growth experiments were conducted for 1h.
The samples were removed from the furnace after the system cooled down to room temperature. Zinc has a high saturation vapor pressure and can be evaporated thermally at low temperatures (about 400oC).Zinc reacts readily with oxygen due to its high chemical activity. Before CVD process and ZnO fabrication, the Si substrate was deposited with gold catalyst via sputtering method. The gold catalyst was deposited with a DC planer magnetron sputtering system, which is shown in (Figure 1b), using a circular 50mm diameter and 2mm thick Au target of 99.998% purity. The target-to-substrate distance was at 3 cm. A continually variable DC power supply of 700V and 15mA was used as power source for sputtering. The substrates were 10 × 10mm2 Si and were ultrasonically cleaned in acetone and ethanol. The substrate temperature was 300°C. The base pressure was lower than 2×10-5 Torr. Pure argon gas was used as sputter gases. With Ar+ bombardment on Au target the gold catalyst was deposited on Si substrate.
Results and Discussion
XRD analysis
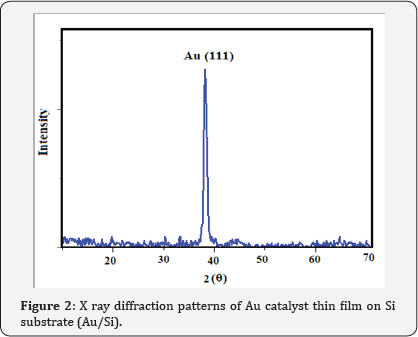
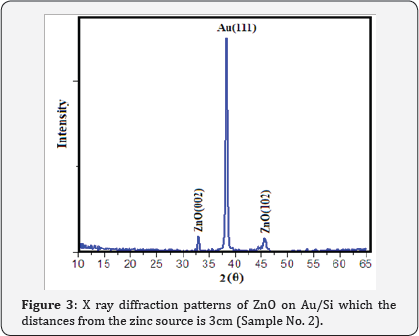
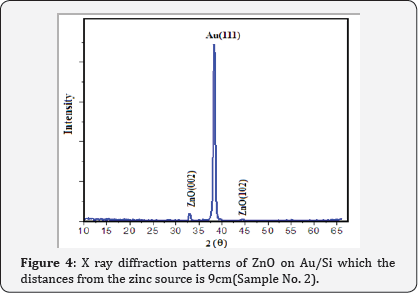
XRD patterns of Au catalyst on Si samples prepared via sputtering method in this work are shown in (Figure 2) From (Figure 2) it is concluded that all the Au catalyst single crystal with a cubic spinal structure which generally occurs in the growth of Au thin films. The XRD pattern has a peak correspond to (111) plane which are compared with JCPD data. (Figure 3 & 4) shows the XRD patterns of deposits formed on Au/Si substrates which are placed at different distances from the zinc source at the furnace temperature of 800oC. The absence of a Zn peak in the XRD pattern demonstrates the absence of Zn atoms in the structure of the nanostructure and completes oxidations formation process. By increasing the distances from the zinc source, ZnO (002) and ZnO (102) peak intensity is decreased. Polycrystalline films deposited on substrates generally show preferred orientation, with a strength which depends on the deposition method, film material, and deposition conditions.
SEM analysis
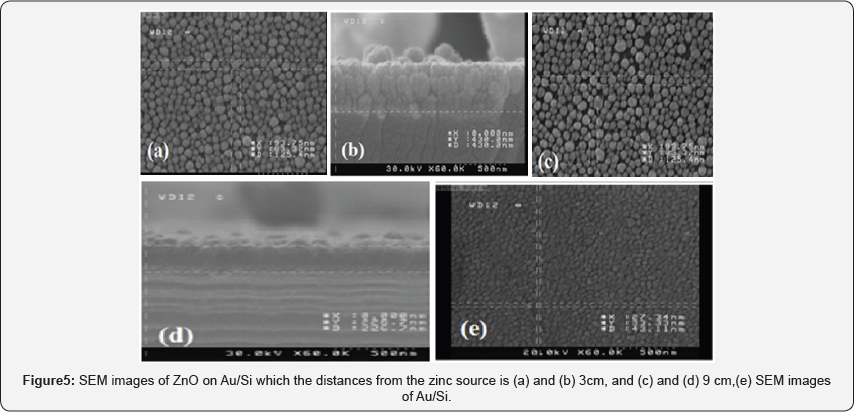
In our work, we reported on the structural characteristics of thin films obtained using SEM analysis. Figure 5a shows the SEM images of the Au catalyst thin film on Si substrate (Au/ Si). The morphology of Au grains is found to be continuous and dense. It is found that the deposit morphology is sensitive to the distance from the source. With decreasing distance from the source, the substrate temperature changes and the morphology of the deposit changes from a polycrystalline film (Figure 5b) to oriented nanorods (Figure 5c, d, e). From cross section SEM images it is indicate that the thickness changes from 400nm to 200nm by increasing distances from the zinc source in CVD system (Figure 6).
Antibacterial property of ZnO nanostructure
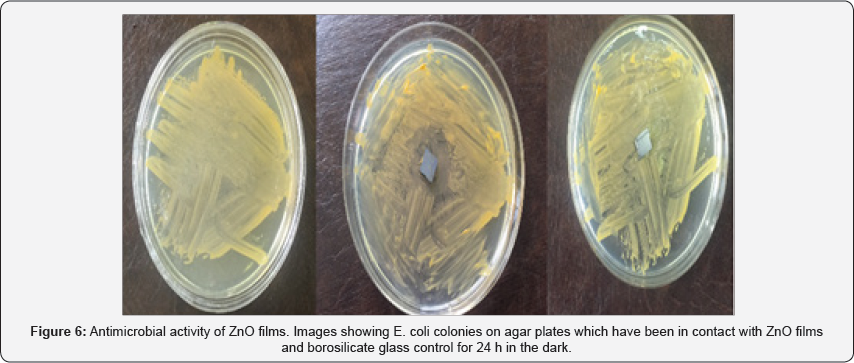
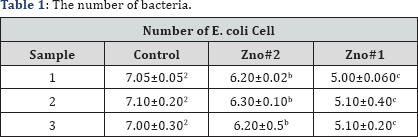
ZnO structures such as silver nanoparticles that have a large surface-to-volume ratio have shown a high degree of antibacterial property [18]. The antimicrobial activity of ZnO-based materials depends on the nanostructure that should provide a high surface-area-to-volume ratio and hence a high fraction of surface atoms [19,20] which enhances the antibacterial property of ZnO. Medium suitable for this test is a nutrient agar. So we estimate the culture medium in terms of number plates. We put the plates in the incubator for 24 h. A fresh shake culture of Escherichia coli was prepared in sterile LB medium by shaking at 37 oC and 200 rpm for 18h. The results of counting the number of bacteria using SPSS software showed that the number of bacteria on the ZnO samples is reduced. It is possible that Zn2+ions react with negatively charged cell wall of E. coli and initiate wall leakage leading to death of bacteria. Antibacterial activity may also be due to production of hydrogen peroxide or hydroxyl radicals caused by ZnO (Table 1).
Conclusion
We produced and obtained the antibacterial effect and morphological properties of ZnO nanostructure with different thicknesses by applying CVD method. Nanostructure and morphology of the produced samples were obtained using XRD and SEM. Antibacterial properties were investigated against E. coli. This also indicates that a ZnO nanostructure could be used as anti-bacterial coating which would be useful in medical applications.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555570.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment