Zinc Nanoparticles: an Investigation of Deleterious Effect on Micro-Organisms-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
Nanotechnology is a thriving field with a number of potential applications and is widely used in the fields of medical sciences like drug delivery [1], bio sensing [2], and in enhancing antibiotic activity [3]. In addition to the various applications in the fields of science and technology, an increasing use of nanomaterials has also been reported in biological and medical applications. In particular, Nano pharmaceutics is one of the disciplines that benefit the most from this technology. The importance of metal nanoparticles has been exploited in almost all the fields. Nanomaterials, with their unique size-dependent physical and chemical properties have shown promising advantages as gene delivery vehicles [4,5], ultra-sensitive intracellular detectors [6] and novel therapeutic drugs [7].
Increasing multidrug resistance of pathogens is one of the foremost concerns facing the medical fraternity and is one of the reasons for the emergence of nanomaterials in the fields of therapeutic sciences. Many of the powerful antibiotics used earlier are increasingly ineffective against these pathogens. Such drug resistant strains of bacteria are called as superbugs and the need for effective antimicrobial to fight these superbugs is undisputed. For a past few decades tremendous investigation has been carried out to develop nanomaterials with unique properties that have specific therapeutic benefits, in particular antimicrobial activity to combat the growing threat of infectious diseases [8,9].
Metals have been documented to exhibit antimicrobial activity for centuries and were widely used before the advent of antibiotics. Antibiotics have replaced in the last few decades all the other antimicrobials because of this efficacy. Antibiotic resistant strains of bacteria have driven us back to metal antimicrobials to confine certain deadly diseases. In addition metal antimicrobials in the nano size are many times more effective than earlier meal based drugs. The use of metal nanoparticles as drug carriers has opened up a new pathway for targeted tumor therapy. Transition metal NPs also find extensive applications as antimicrobial and antifungal agents in textile [10], food and cosmetic industries.
Among the various metal nanoparticles studied for their antimicrobial properties, Zn NPs have found to be more effective against the tested bacterial strains [11]. Zinc is a ubiquitous essential metal ion and plays a role in catalysis, protein structure and perhaps as signaling molecules in living systems hence, even the low concentrations of Zn NPs have better antimicrobial effect. Zinc is known to play a central role both in vivo and in vitro in immune system as severe zinc deficiency (even a moderate zinc deficiency) is known to affect the immunity in the human system. Zinc has structural and gene-regulatory functions in living organisms and participates in catalysis of many essential metabolic reactions. However, at super physiological levels, zinc inhibits the growth of many bacteria which makes it a better antibacterial agent against pathogenic bacteria.
Zn NPs kill the bacteria by directly interacting with the bacterial membrane and hence presented at high concentrations of Zn NPs can damage the DNA and the membrane wall beyond repair effectively killing the bacteria [12-14]. Zn NPs inhibit the growth and kill both gram-positive and gram-negative bacteria, and specifically staphylococcus aureus and Escherichia coli leaving the human cells unharmed. Staphylococcus aureus has been a growing problem in the field of medicine, as new antibiotic resistant super strains of such bacteria have emerged.
It has been experimented that, increased antibacterial effect of Zn NPs against the pathogens can be observed as the particle diameter is reduced. However, it was observed that, the concentration of Zn NPs is a more important factor than the size and shape of the NPs for antibacterial effects. Zinc ions are effective antimicrobial agents even at low concentrations. Zinc is well known for its antiviral property against the common cold causing virus known as rhinovirus. It could reduce the severity and duration of common cold. The rhinovirus attaches itself to the linings of nasal cavities and infiltrates the cells by attaching to a cell receptor called Intercellular adhesion molecule- 1(ICAM-1). Zinc ion blocks the ICAM-1 receptor, thus preventing the rhinovirus from entering cells and inhibits the common cold infection.
Zinc - Biological role: review
A special position among metals is occupied by the redox inert metal zinc. Zn is the second most common trace metal in the human body and plays multiple roles in the biological system which can be differentiated into three main categories as catalytic, regulatory, and structural. Zn is required for the catalytic activity of more than 200 enzymes in our body, and mainly needed for the structural integrity of proteins and regulation of gene expressions (a component of DNA transcription proteins, without which DNA could not be copied). It was demonstrated that, depletion of zinc may enhance DNA damage via impairments of DNA repair mechanisms. It is also an essential micro-nutrient for prokaryotic organisms. Zn is also required for proper sense of taste and smell [15].
Zn is a major intracellular regulator of lymphocyte apoptosis in vitro and in vivo. Zn status affects numerous lymphocyte functions including mitogenesis, antibody synthesis and other facets of cell-mediated immunity, which are negatively impacted by Zn deficiency. Zn deficiency in human is known to be an important malnutrition problem and it was first discovered in 1961. Zn deficiency was discovered by acrodermatitis enteropathica (a rare autosomal recessive inheritable disease) as a Zn-specific mal absorption syndrome. This disease is also accompanied by thymic atrophy resulting in an immune defect and a high frequency of bacterial, viral and fungal infections. Epidermal, gastrointestinal tract, central nervous system, immune system, skeletal and reproductive systems are the organs most affected clinically by Zn deficiency.
Zinc possesses antioxidant properties which may protect against accelerated aging and helps speedup healing process after injury. Zinc is an essential component of numerous proteins involved in the defense against oxidative stress. Zinc helps maintain the integrity of skin and mucosal membranes. It is mostly confined in muscles and bones to support normal growth and development during pregnancy, childhood, and adolescence. It was successfully used in the treatment of acrodermatitis, enteropathica, Wilson's diseases, gastrointestinal disorders, infertility and so on. In addition Zn has an impact on the immune system and possesses Neuro protective properties.
Zinc plays an important role in axonal and synaptic transmission and is necessary for nucleic acid metabolism and brain tubulin growth and phosphorylation [16]. Wound healing effect of zinc containing ointments has been known for centuries. Zinc may be used as a therapeutic agent and it may act as a play role in the prevention of pain crisis in sickling cell disease. Zn NPs were essentially synthesized by novel electrochemical technique as described in our previous work [17]. The electrodeposited Zn NPs were characterized using by HR-SEM, HR-TEM, UV-diffuse reflectance spectroscopy and FT-Raman spectroscopy for their optical properties.
Experimental
Materials and methods
ZnSO4.7H2O (99.8% pure) and hydrazine sulphate (99.5%) of analytical grade was purchased from M-merck, and used as received without further refinement. An electrochemical cell consist of Zinc plate (99.9%) of 60 cm2 and Glassy carbon electrode purchased from Sigma Aldrich were used as working electrode and counter electrode respectively for the electrolysis process. Double distilled deionized water obtained from M-merck was used for the electrolyte preparation. During electrolysis both the electrodes were held parallel to each other at a distance of 3cm. The process was carried out Galvano statically at room temperature with 10mA current drawn from regulated direct current power supply unit. The pH of the electrolyte was maintained at 3.2 throughout the process with hydrazine sulphate concentration. The bacterial strains like Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Klebsiella Pneumonia were used in this study.
Electrochemical synthesis of Zn NPs
Zn NP was synthesized from the novel electro deposition method using zinc sulphate and hydrazine sulphate as described in our previous work. An electrolyte consisting of 0.02M of zinc sulphate and 0.005 M hydrazine sulphate were prepared in double distilled deionized water. The process was carried out for 90min and at the end of the process the matte grey deposit obtained at the cathode surface was washed with milli-Q water, dried and carefully scrapped using a nickel knife.
Characterization
The morphological features like size and shape of the electrodeposited Zn NPs were characterized by FE - SEM working at an accelerating voltage of 120KV. TEM images were obtained with a JEM-1400 High Resolution Transmission Electron Microscope. Raman measurements were carried out using Bruker RFS 27: standalone FT-Raman spectrometer with resolution 2cm-1. The purity of the Zn NPs was measured by the FT-IR spectroscopy. The surface plasma on resonance property of Zn NPs was analyzed using diffuse reflectance spectrometer in the reflectance mode. The effect of surface charge of the nanoparticles was calculated from the zeta potential measurements.
Antibacterial propensity
Zinc nanoparticles were screened for the antibacterial activity against the bacterial strains like Methicillin-resistant Staphylococcus aureus, Escherichia coli, Enterococcus Faecalis and Pseudomonas Aeruginosa were purchased from Institute of Microbial Technology (IMTECH), Chandigarh, India. Zinc NPs were tested for antibacterial effect against gram positive and gram negative bacterial strains, evaluated by micro dilution method. Zn NPs showed an excellent antibacterial activity against the tested pathogens.
Results and Discussions
FTIR spectroscopy
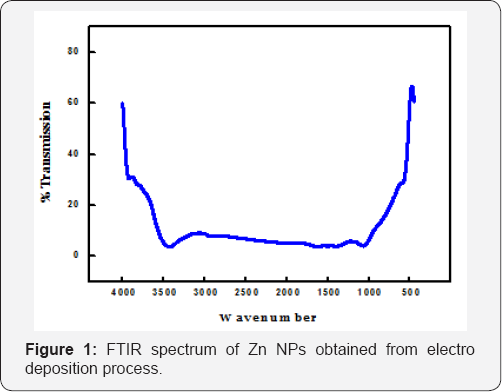
Fourier Transform Infrared spectroscopy studies of Zn NPs was carried out using Thermo Avtar 370 at ambient temperature to delineate the purity and crystalline nature of the electrodeposited Zn. FTIR characterization revealed the fact that, the electrodeposited Zn NPs has not oxidized to a molecule since no polar bond vibrations are seen in the spectrum. Figure 1 shows the FT IR spectra of Zn NPs obtained by electro deposition from the sulphate bath at optimum pH. The broad absorption peak observed at 3428cm-1 and a very small absorption bend at 3921cm-1 in the spectrum were assigned to O-H stretching vibrations of water molecule adsorbed on the surface of the metal particles during sample preparation. The vibrational peaks obtained in the fingerprint region between 400cm-1 to 1000cm-1 in the spectrum are due to inter-atomic skeletal vibrations since metals generally give absorption bands in fingerprint region.
FT - Raman spectroscopy
Raman spectroscopy is the preferred tool that is used predominantly for the determination of size of the nanoparticles. The spectrum obtained from Raman measurement can be used in a number of ways where the simplest one is to use it as a finger print for identification of species and crystal phases. Raman spectrum was applied to analyze crystalline quality, surface condition and homogeneity. Crystalline sample will have broad Raman peaks and these Raman peaks were attributed to acoustic phonon modes restrained in homogeneous particles. The particle size measured XRD and SEM measurements are in very good agreement with estimated size of Zn NPs from the photon confinement model using the position of the Raman peak.
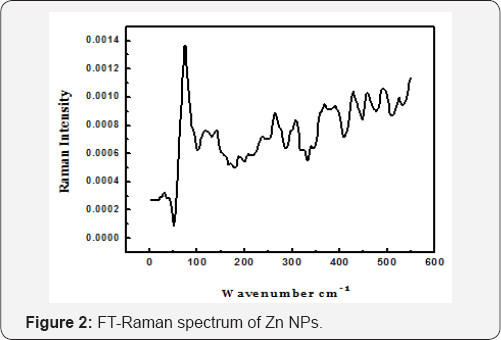
Raman spectra of Zn NPs were recorded by a triple mono-chromate equipped with a photon counting system. The 514.5 nm line of Ar-ion laser was used to excite the spectra. All measurements were carried out at room temperature. Most of the metal crystals have no optical vibration modes, on the other hand hexagonal close packed (hcp) metals like Be, Mg and Zn have vibration modes which are Raman active. Zn NPs consist of a hexagonal crystal structure with space group p63/mmc. For zinc F2g mode normally lies at 70cm-1 and this F2g mode for the electrodeposited Zn NPs was found crystalline nature of the sample and is shown in (Figure 2). The shift in peak position indicates that the Zn particles obtained are in nano scale regime.
Diffuse Reflectance Spectroscopy
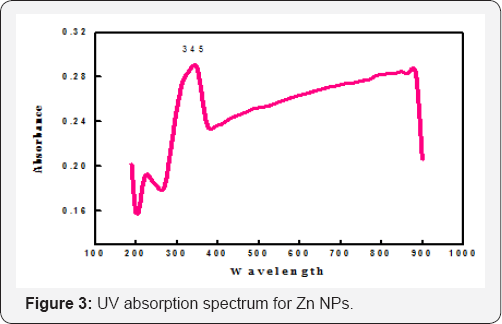
Diffuse reflectance is an exceptional tool for characterizing the crystalline material in the mid-IR and NIR region. Reflectance spectrum of the electrodeposited Zn NPs was characterized by Perkin Elmer Lamda-2 spectrophotometer and the surface plasm on resonance peak is shown in (Figure 3). The peak position and sharp intensity of the absorption spectrum confirmed the presence of Zn NPs and it is in good agreement with the SEM investigations of the Zn NPs. The surface plasm on resonance band was detected near the blue shift at around 345 nm which indicates the particles obtained are in the nanoscale. The shape of the absorption peak suggests the anisotropic nature of the Zn NPs (hexagonal in shape) and is confirmed by the electron microscopy images.
Optical band gap
Optical properties of metal nanoparticles in their solid state are frequently determined through UV-Visible spectroscopy of their dispersed solution in liquid medium. Though the peak position of the absorption band of the nanoparticles could not define well from these techniques, the precise determination of the band gap energy is difficult. The band gap of the material is associated with the electric conductivity of that material. There is generally no band gap in metals, but the band gap value for insulators are generally large, and for semiconductors the electrons with minimum thermal energy can jump from the valence band to conduction band and the optical band gap is typically intermediate between the metal and insulator. However, using Kubelka-Munk treatment on diffuse reflectance spectra it is possible to extract the band gap unambiguously.
The optical band gap energy for the Zn NPs was calculated using Tauc Equation;
(αhν)Λn=A(hν-E_g ),
Where, A is a constant, h is the plank's constant, E_g is the band gap, ν is the frequency, and the value of the exponent ndenotes the nature of the sample transition and is equal to % for the allowed direct transition and athe absorption coefficient. The value of ain the Tauc equation is calculated from the diffuse reflectance using Kubelka-Munk equation,
F(R_∞)=(1-R)Λ2/2R=k/s - (2)
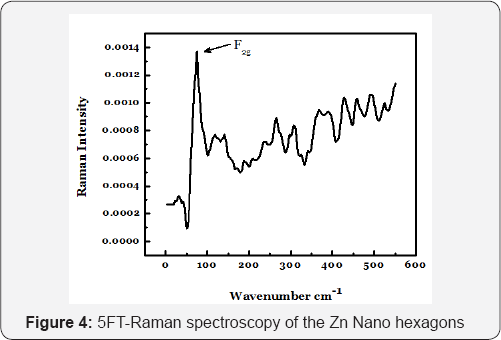
F(R_∞) is the so-called re-emission or Kubelka-Munk function, where R_∞=R_sample/R_standard . The observed diffuse reflectance spectrum is converted to F(R), which is equivalent to the absorption coefficient (α). Where, R is the absolute reflectance, k is the molar absorption coefficient and s is the scattering coefficient. The curve between the quantity [F(R)hv]Λ(0.5) on the horizontal axis and photon energy (hv) on the vertical axis is drawn. A line is drawn tangent to the point of inflection on the curve and the band gap value is obtained by the intercept of the fitted straight line on the horizontal axis. The value of optical band gap energy for the electrodeposited Zn NPs was found to be 2.8 eV and is shown in the (Figure 4).
Electron microscopy characterization

The detailed structural characterization was done by HR- SEM. Surface morphology of Zn NPs was characterized by HR-SEM (FEI Quanta FEG 200). HR-SEM images of the Zn NPs obtained from sulphate bath revealed valuable information relating to the purity of a nanoparticle sample as well as an insight on their degree of aggregation. From the SEM images, it is realized that the crystalline morphology was pure in composition. Furthermore, the SEM micrographs clearly show the high grade of dispersion and uniformity of Zn NPs. HR- SEM images of Zn NPs obtained with and without additives at optimized conditions of the electrolyte is shown in (Figure 5).
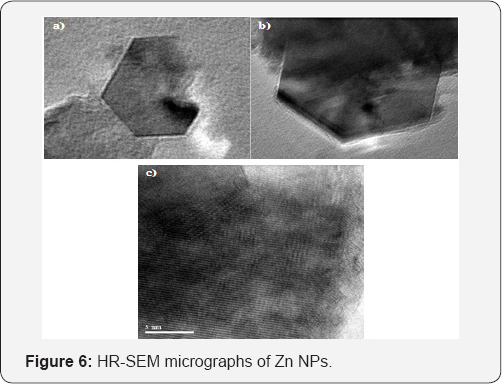
The most important information about Zn NPs is the size, shape and their distribution, which crucially influence their properties on bactericidal action. High Resolution Transmission Electron Microscopy (HRTEM) can yield information such as morphology, particle size and size distribution of the nanoparticles. In this technique the individual particles are directly observed and measured. HR-TEM images of Zn NPs clearly indicated the presence of hexagonal surface morphology of zinc. Lattice fringe images obtained from HR-TEM, that can be directly related to the structure and interpretation has confirmed the defects free zinc crystal and interfaces at atomic scale resolutions as shown in (Figure 6).
Antibacterial activity
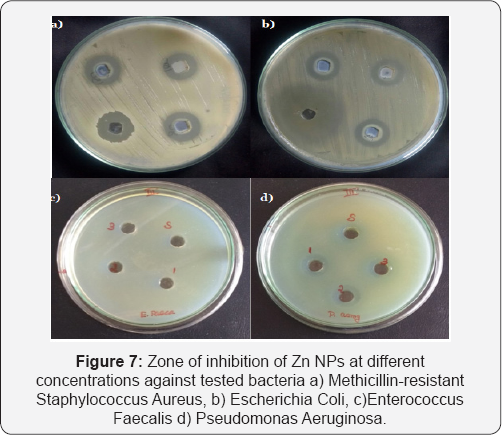
The antibacterial propensity of the electrodeposited Zn NPs was tested against gram-positive and gram-negative bacterial strains by Kirby-Bauer well diffusion method using Muller-Hinton agar (MHA) medium. The bacterial growth was determined by measuring the diameter of inhibition zone and the results obtained indicate that the Zn NPs have better antibacterial effect. Zone of inhibition was measured after 24 h of incubation at room temperature and the results are shown in (Figure 7).
The mechanism of antibacterial action of Zn NPs against the microorganism is not evident, however the antibacterial propensity of Zn NPs could be understood by the membrane damaging insensitivity. Zinc ions are known to inhibit multiple activities in bacteria such as glycolysis, transmembrane proton translocation and acid tolerance [18]. Thus the presence of zinc ion is possible to inhibit proliferation of bacterium by binding to the membrane of bacteria, which can prolong the lag of growth, and contribute to the antimicrobial activity of zinc. It has been found that, zinc gave a rapid impact on bacterial productivity; however proteolytic enzymes were not affected [19]. Zn NPs kill the bacteria by directly interacting with the bacterial membrane. It has been shown that high concentration of Zn NPs can damage the membrane wall beyond repair effectively killing the bacteria.
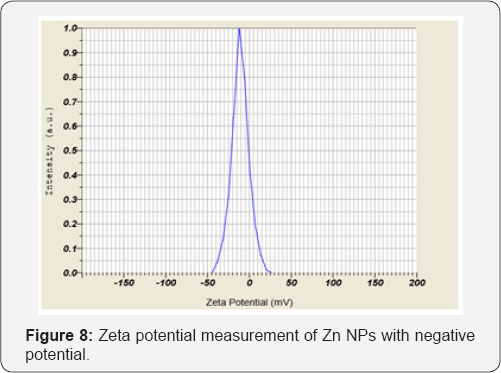
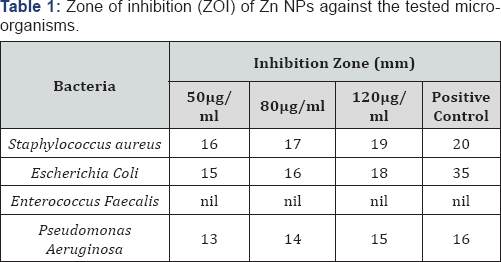
The columbic repulation forces induced by surface negative charge minimize the aggregation and this confirms the stability of the nanoparticles. Figure 8 shows zeta potential values obtained for electrodeposited Zn NPs with surface potential of -11 mV. There are electrostatic interaction between NPs having opposite zeta potential and cell surface as the crucial step towards nano toxicity followed by cell morphological changes, increase in membrane absorptivity and their accumulation in the cytoplasm. The toxicity of Zn NPs is more evident on gram positive bacteria compared to gram negative bacteria. Due to additional layer of negatively charged lipo polysaccharides, Gram negative bacteria are more negatively charged than Gram positive bacteria. Interestingly, the bactericidal action against Enterococcus faecalis showed no result, as a well-known fact that E. Faecalis is resist to most commonly administrated antibiotics. These results indicate that, Zn NPs have excellent antibacterial action against the pathogenic bacterial strains on the other-hand non-toxic to bacterial flora.
The lowest concentration of Zn NPs that completely inhibits the visible growth of the microorganism is recorded as minimum inhibitory concentration (MIC). MIC of Zn NPs against the tested pathogens was recorded and is shown in table. The MIC values for the Zn NPs were estimated from the zone of inhibition diameter. The results showed that zinc nanoparticles have wonderful bactericidal activity against the gram positive bacterial strains. The bacterial activity increases with increasing nanoparticle concentration. In case of gram negative bacteria this is known for low concentration due to the presence of negatively charged cardiolipins on their cell surface.
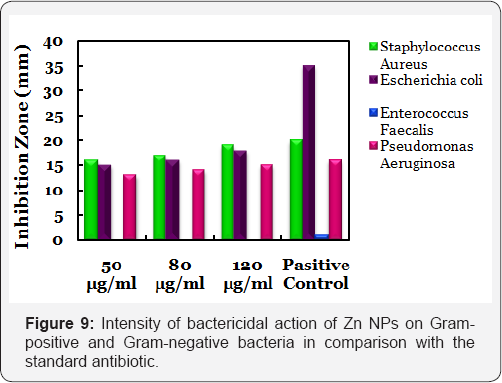
Electrodeposited Zn NPs with negative surface potential exhibited very high antibacterial activity with MIC 50 for both Gram-positive and Gram-negative bacterium and the comparative result with standard antibiotic (Ampicillin) is indicated in Figure 9. Zn NPs with negative surface potential showed significant antimicrobial propensity against the Gram-positive bacterium. The antimicrobial efficacy of the Zn NPs was comparable with the commercially available antibiotic drug ampicillin. The zone of inhibition for Zn NPs against the tested bacteria is given in Table 1.
Conclusion
Zinc Nano hexagons were synthesized by electro deposition method without any nanoparticle stabilizers. A blue shift of the band edge for the Zn NPs showed the influence of the reaction conditions. Plasmon absorption of Zn NPs shows that they are the potential candidates for wide band gap (2.8eV).The FTIR spectrum showed that the Zn NPs obtained are free from impurities. The results obtained from the antibacterial tests suggest that Zn NPs can find applications in the field of medicine, pharmaceuticals and food industries. At higher concentration, the Zn NPs showed better bactericidal effect towards the Grampositive bacteria compared to Gram-negative bacteria. The results obtained suggest that Zn NPs can find applications in the field of medicine, pharmaceuticals and food industries.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555569.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment