MgO nanostructures: Formation and Antibacterial Activity-Juniper publishers
Global Journal of Nanomedicine-Juniper publishers
Introduction
While nano materials have been generated by physical methods, chemical approaches have proved to be more effective and efficient as they provide better control over the size and shape, which is one of the essential features of nano materials. Chemical synthesis of nano materials has been reviewed by few authors but innumerable improvements and better methods are being reported continually [1]. The chemical methods reported in the literature include decomposition routes of magnesium precursor salt, sol-gel and solvo thermal process [2-5].
These approaches generally make use of frequent toxic organics in presence of harmful gases at very high temperatures that can produce unintentional defects and the product can rarely be used for medical applications. Recently, an interesting and encouraging result provides motivation to synthesize magnesium oxide nano particles without additives at low temperatures and test their antibacterial activity [6].
Nano-MgO is a functional material that has been widely used in various areas and very recently it has been reported that MgO has a good bactericidal performance in aqueous environments [7]. Work by Klabunde & Ahran [8] demonstrated that nano- MgO exhibits high activity against bacteria, spores and viruses because of its large surface area, positively charged particles which can result in strong interaction with negatively charged bacteria. Compared with TiO2, silver, copper and other kinds of solid bactericides, nano-MgO has the advantages of non toxicity of being prepared from readily available and economical precursors and therefore has considerable potential as a solid bactericidal material [9,10].
In this work, nano-MgO particles with different particle size were prepared using a versatile and non toxic technique. The expedient process to synthesize MgO nano particles by a simple reaction of magnesium foil with de-ionized water without use of organics/amines is reported. The nano particles so obtained have diameters ranging from 45 to 75nm. The synthesized products were characterized in terms of their structural properties. The effect of as synthesized particles was investigated. The bactericidal efficacy of the nano-MgO against both bacteria. Systematic studies would be necessary to optimize the conditions for obtaining nano particles of desired dimensions.
Experiments
Experimental materials
Magnesium foil (Ranbaxy Chemicals) has been used without any preheated producer or any further purification and deionized water has been prepared in our laboratory. For the synthesis of nanoparticles, a closed cylindrical Teflon lined stainless steel chamber of 50ml capacity was used.
Preparation of MgO
In a typical synthesis, 2 mg of magnesium powder was taken in a vial containing 20 ml of de-ionized water and was well sonicated for 10 minutes before placing at desired temperature in a Teflon bomb of 5o ml capacity. The reaction mixture was transferred to teflon-lined stainless steel chamber and has been kept at 125°C in an oven for 7h. After the desired time, the system was naturally cooled to room temperature. The reaction mixture was centrifuged to reclaim the precipitated sample and was washed with distilled water. After drying in air, the final white powder was obtained.
Characterization
The microstructure and possible chemical composition of the as synthesized sample were examined by FE-SEM (FEI NOVA NANOSEM-600) coupled with energy dispersive x-ray spectrometer (EDX). The morphology was further checked by TEM.Bactericidal testing
After being heated at 125°C for 10 minutes, 0.1 g samples of MgO prepared were placed in a sterile conical flask, to which was added 50ml phosphate buffer (pH. 6.8) followed by 0.5ml germ. After the mixture was mechanically shaken for 2h at 37°C, an aliquot (0.5ml) of the liquid mixture was placed on a sterile plate with a general germ culture medium and cultured for 48h at 37°C. The surviving colonies were counted with eyes under the help magnifier and the bactericidal efficacy was calculated according to the following formula:
Bactericidal efficacy (%) = alive number in reference group- alive number in experimental group/alive number in reference group x 100%
Test plates were contacted with B.subtillis or S.aureus at 37°C for 24 h under sunlight or in the dark. After counting the number of living spores and living bacteria by the colony counts method proposed in the literature [12], the bactericidal efficacy was calculated according to the formula.
Results and discussion
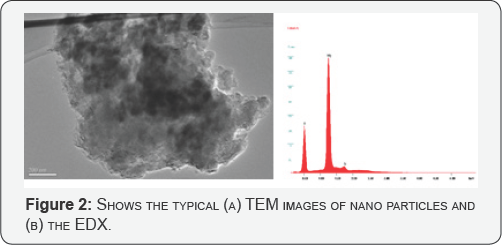
For the micro-structural analysis, the as-synthesized samples were directly transferred to the FESEM chamber without disturbing the original nature of the products. (Figure 1 a &b) show the low and high magnification FESEM images of the nanoparticles and confirms that the nanoparticles are grown in a very high density. The typical diameters of the as-grown nano particles are ~ 60 ± 15 nm. A closer view reveals that most of the nano rods have varying diameter. The morphology was further confirmed by TEM (Figure 2). To check the composition of the as-grown nano particles, EDX analysis was performed. (Figure 2) demonstrates the typical EDX analysis of the as-grown MgO nano particles. Except Mg and O, no other peak for any other element has been found in the spectrum which again confirmed pure MgO.

Microbiology Testing
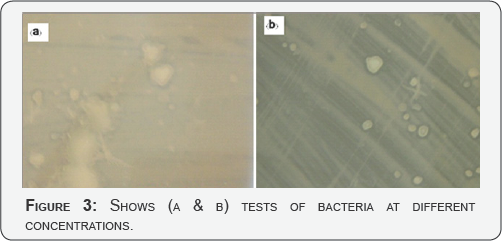
A class strain of E. coli represented gram-negative bacteria, while S.aureus was used as a gram-positive bacterium. Both bacterial strains are fairly robust and resist disinfection. The fundamental difference between gram negative and gram positive bacteria is the nature of the cell wall. Gram positive bacteria have an additional thick peptidoglycan layer that appears purple when testing treated with crystal violet during gram stain. Gram negative bacteria have additional outer membranes but a much thinner peptidoglycan layer between its inner and outer membranes. The drastic difference in the nature of the cell boundary in contact with the environment often dictates the basic response of the cell to environmental forces such as anti microbial agents (Figure 3 a & b).
In our hydrothermal approach, reaction temperature, time and pressure plays a crucial rule for the growth and formation of nano rods. The earlier techniques involving a hydrothermal hydrolysis to produce Mg (OH)2 and subsequent thermal decomposition to obtaining MgO nano rods has been well explained. Comparing with those methods using MnCl2 as precursor or using a mixture of MgO powder and carbon powder as starting materials, Mg is the only reactant in this technique, which is more direct and easy.
Conclusion
We are describing a new and a versatile route for the synthesis of MgO nano particles using de-ionized water as a solvent without using any organics. Since the pure water is used as solvent as well as the source of oxygen and the approach is believed to be non toxic without producing hazardous waste. Compared with other methods, the present method is fast, economical, low temperature and free of pollution which will make it suitable for large scale production. The effect of as synthesized particles was investigated. The bactericidal efficacy of the nano-MgO against both bacteria. Systematic studies would be necessary to optimize the conditions for obtaining nano particles of desired dimensions.
For More Articles in Global Journal of Nanomedicine
https://juniperpublishers.com/gjn/GJN.MS.ID.555566.php
Please Click on: https://juniperpublishers.com/gjn/index.php
For More Open AccessJournals In Juniper Publishers Please Click on: https://juniperpublishers.com/index.php

Comments
Post a Comment